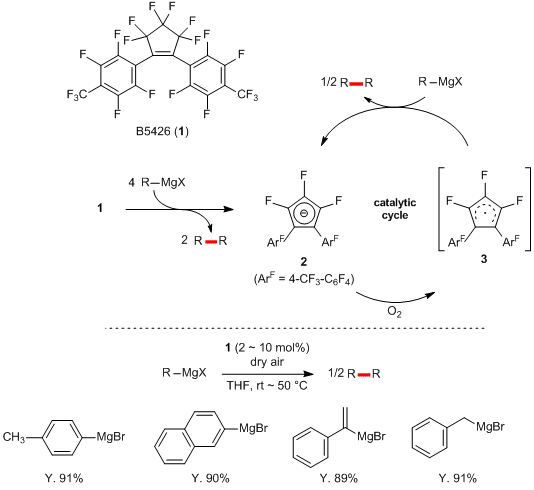
Highly fluorinated cyclopentene derivative (
1) was developed by Korenaga
et al. and can be used for oxidative homocoupling of Grignard reagents. By using
1 as a catalyst, oxidative homocouplings of aryl Grignard reagents, vinyl Grignard reagents and benzyl Grignard reagents proceed in high yields. In this reaction,
1 functions as a four-electron oxidant initially and is converted into the anion species
2 with dimerization of four molecules of the Grignard reagent. Subsequently, an air oxidation of
2 affords a radical species
3, which catalyzes dimerization of a Grignard reagent with regeneration of the anion species
2 to complete the catalytic cycle. In this way,
1 is expected to be used as a green catalyst for homocoupling of Grignard reagents using air as a co-oxidant.