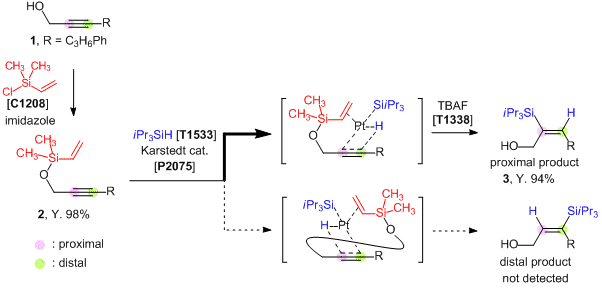
Chlorodimethylvinylsilane (DMVS-Cl) is a useful directing group introducing agent for the regioselective hydrosilylation of alkynes. Hydrosilylations are commonly used as a way to synthesize silyl alkenes but it has been difficult to control the regioselectivity (proximal vs. distal) in unsymmetrical alkynes system. Tomooka
et al. have found that the dimethylvinylsilyl (DMVS) group acts as an excellent directing group of hydrosilylations for the high regioselectivity. The DMVS group can be introduced into propargyl or homopropargyl alcohols by using DMVS-Cl as a reactant and the consequent hydrosilylation of the formed DMVS group-substituted alkynes proceeds with the substitution of silyl groups at the proximal carbon. After the reaction, the DMVS group can be removed by treatment of TBAF.