Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Bitte wählen Sie die Menge aus.
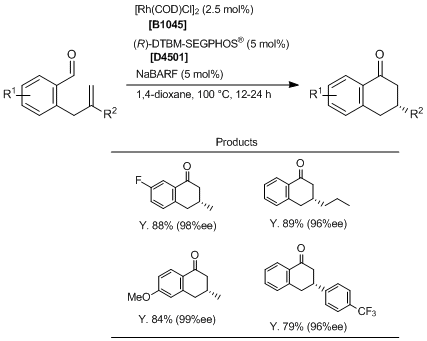
Rh-catalyzed endo- and Enantioselective Hydroacylation of o-Allylbenzaldehydes
Stanley et al. have reported Rh-catalyzed endo- and enantioselective hydroacylation of o-allylbenzaldehydes. According to their results, by using [Rh(COD)Cl]2, (R)-(-)-DTBM-SEGPHOS® and NaBARF as a catalyst system, the desired hydroacylation reaction proceeds endo-selectively to generate 3,4-dihydronaphthalen-1(2H)-one products with high yields and enantioselectivities while minimizing the formation of byproducts from competitive alkene isomerization and progress of ene/dehydration pathways. exo-Selective NHC-catalyzed hydroacylations have been also reported by Glorius et al. in recent years, so this reaction can be used complementarily.