Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
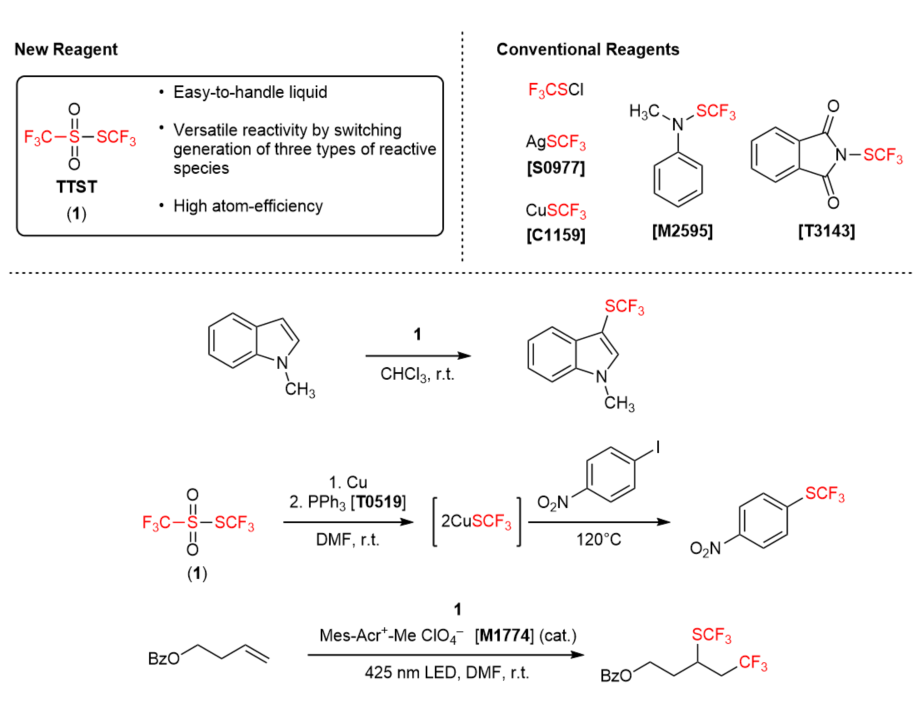
Versatile Trifluoromethylthiolating Reagent: TTST
Trifluoromethylthio group is known to have a remarkably large hydrophobic parameter among fluorine-type substituents.1) Therefore, various methods and reagents have been developed to improve the properties of target compounds in drug discovery research. Nevertheless, there are still some problems like stability and versatility.2)
TTST (1) is a novel trifluoromethylthiolating reagent developed by Umemoto et al.3) 1 is easy-to-handle because 1 is a stable liquid in air. Furthermore, 1 shows highly versatile reactivity. By choosing suitable reaction conditions, three types of reactive species (cations, anions and radicals) can be generated. Therefore, it can be used for versatile reactions such as electrophilic trifluoromethylthiolation of electron-rich aromatic rings and reactive methylenes, Cu-mediated nucleophilic trifluoromethylthiolation of aryl iodides and radical trifluoromethylthiolation of alkenes. In addition, because the ratio of the available group (-SCF3) in 1 is high, it is advantageous that fewer wastes are derived from 1 compared to conventional reagents and that 1 has high atom-efficiency.
References
- 1) "Aromatic" Substituent Constants for Structure-Activity Correlations
- 2) Synthetic Methods for Compounds Having CF3−S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions
- 3) Preparation and Reactivity Study of a Versatile Trifluoromethylthiolating Agent: S-Trifluoromethyl Trifluoromethanesulfonothioate (TTST)
Related Products
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.


