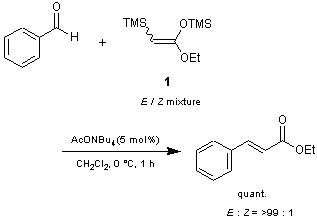
Mukaiyama and Michida have reported that E/Z mixtures of ketene acetals 1 and 2 react with various kinds of aldehydes in the presence of a catalytic amount of acetate salts to give α,β-unsaturated esters in high yields with excellent stereoselectivities.1) This procedure has some advantages over conventional methods such as the Peterson reaction and the Horner-Wadsworth-Emmons (HWE) reaction. For example:
1) Catalytic reaction (with no need of equimolar amount of base); 2) Co-product (TMS2O) can be removed easily by evaporation; 3) High stereoselectivity is obtained by the proper choice of the counter cation of the catalyst.
Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [Product Highlights] Cell Culture-Ready Antibiotic... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)