TCI Chemistry News
Maximum quantity allowed is 999
Please select the quantity
September 2023
TCI offers an extensive catalog of over 30,000 high quality organic reagents suitable for benchtop-to-bulk chemistry. This issue covers our chemistry topics as follows:
- New Reagents for Fluorination: Umemoto Reagent IV and NFBB
- Reagent for the Synthesis of gem-Disubstituted Cyclopropanes
- Oxidation Reaction Using Triphenylantimony as a Catalyst
- Buchwald-Hartwig Amination Using Pd2(dba)3 and tBu3P·HBF4
- Printed Magazine: TCIMAIL 193
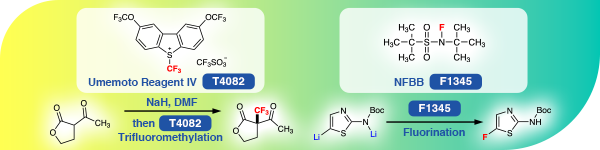
New Reagents for Fluorination: Umemoto Reagent IV and NFBB
TCI offers an electrophilic trifluoromethylating agent, Umemoto Reagent IV [T4082] and an electrophilic fluorinating agent, NFBB [F1345], developed by Umemoto et al. Umemoto Reagent IV exhibits higher reactivity than conventional trifluoromethylating agents such as Umemoto Reagent I and II due to the electronic effects of two trifluoromethoxy groups. In addition, Umemoto Reagent IV is a stable solid, easy to handle in the atmosphere and has low hygroscopicity. NFBB is sterically protected by a bulky tert-butyl group and designed to suppress degradation by strong bases such as organolithium species. The sulfonimide anion generated in the reaction system acts as a deprotonating agent so that the amount of base required to fluorinate active methylene compounds can be reduced to a catalytic amount.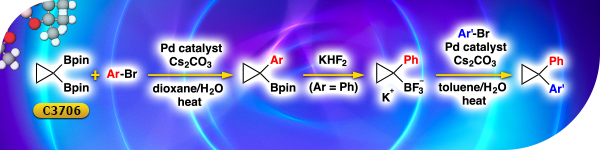
Reagent for the Synthesis of gem-Disubstituted Cyclopropanes
Cyclopropane-1,1-diboronic acid bis(pinacol) ester [C3706] can be utilized in the Suzuki-Miyaura coupling with aryl bromides to synthesize tertiary arylcyclopropylboronic esters. When aryl moiety is a phenyl group, the tertiary arylcyclopropylboronic esters can be converted to arylcyclopropyltrifluoroborate salts with potassium hydrogenfluoride, and then converted to gem-disubstituted cyclopropanes by the subsequent Suzuki-Miyaura coupling.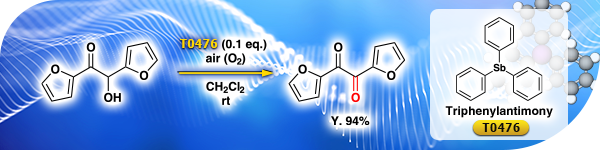
TCI Practical Example: Oxidation Reaction Using Triphenylantimony as a Catalyst
We are proud to present the air oxidation of furoin to furil catalyzed by triphenylantimony [T0476].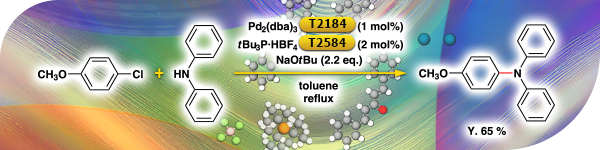
TCI Practical Example: Buchwald-Hartwig Amination Using Pd2(dba)3 and tBu3P·HBF4
TCI is proud to introduce the synthesis of 4-methoxytriphenylamine via Buchwald-Hartwig amination of 4-chloroanisole and diphenylamine by using Pd2(dba)3 [T2184] and tBu3P·HBF4 [T2584] as catalysts.
Printed Magazine: TCIMAIL 193
Released in August 2023, TCI's latest issue features Prof. Hiroshi Sugiyama and coworkers’ article and Prof. Nagatoshi Nishiwaki’s chemistry chat. We also feature research on:
- Air-Stable Ni(0) Complex Ni(COD)(DQ), a Useful Precatalyst
- Naphthalene Carboxylic Anhydride for the Synthesis of Functional Materials
- Graphitic Carbon Nitride and Heptazine-Based Building Blocks
- Measuring Relative Cell Number with Our ATP-Luciferase Assay Reagent
- Hemagglutinin: Recombinant Lectins
- c-Jun N-Terminal Kinase (JNK) Inhibitor
- Activin Receptor-Like Kinase (ALK) 4/5/7 Inhibitor
How to Order TCI ProductsTo order, please contact your local distributor found below.» Our distributors |
| * The prices displayed in the landing website are USD based catalog prices. TCI has specific website and local currency based price list for customers in South Korea, Singapore, and Australia. For customers in other Asia Pacific countries / regions, please visit our Asia Pacific Website and contact our local distributors for the end user's cost. (The USD prices that are shown in Asia Pacific websites are TCI's catalog prices.) Please visit each country/region's website from the following links and confirm the prices. https://www.TCIchemicals.com |
|
If you are interested in our biweekly e-enewsletter "TCI News" and want to receive regularly, please click the link below and sign up. TCI News Sign-Up |
|
Tokyo Chemical Industry Co., Ltd. (TCI) Global Business Department Customer Support and Technical service » globalbusiness@TCIchemicals.com » https://www.TCIchemicals.com |
