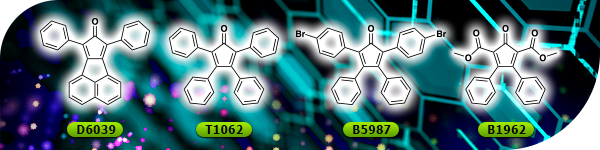
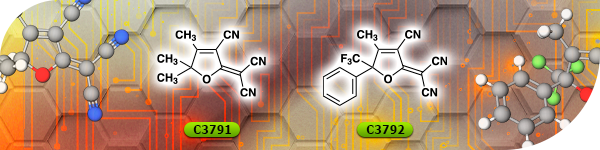
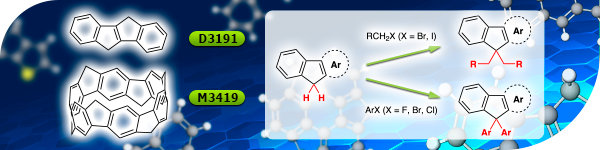
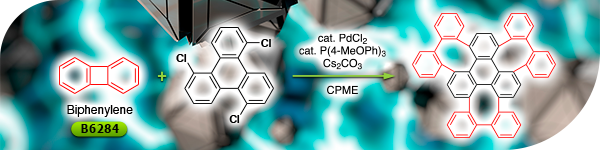
TCI Materials Science News
Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [TCIPracticalExample] Suzuki-Miyaura Coupling Using Encapsulated... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)