TCI Chemistry News
Maximum quantity allowed is 999
November 2022
The Nobel Prize in Chemistry 2022 was awarded jointly to Carolyn R. Bertozzi, Morten Meldal and K. Barry Sharpless "for the development of click chemistry and bioorthogonal chemistry." TCI provides a wide range of click chemistry and bioorthogonal chemistry products. This newsletter shows TCI brochures and articles related to asymmetric catalysts as follows.
- Conjugation Chemistry / Click Chemistry
- Ligands Accelerating Click Reaction
- Copper-free Click Reaction Linkers
- TCI Click Chemistry Articles
Please also see TCI product brochure “Click Chemistry”.

Conjugation Chemistry / Click Chemistry
"Click Chemistry" is a term which was first coined by K. B. Sharpless in 2001 to refer to reactions that afford products in high yields and in excellent selectivities by carbon-hetero bond formation reactions. The term "Click" means joining molecular pieces as easily as clicking together the two pieces of a seat belt buckle. In general, the definition of click chemistry is described as follows: (1) give very high chemical yields of desired products, (2) combination of readily available simple building blocks, (3) generate almost no byproducts, (4) simple product isolation by non-chromatographic methods, (5) reaction proceeds in water, as well as in organic solvents.
While there are a number of reactions that fulfill this criteria, the Hüisgen 1,3-dipolar [3+2] cycloaddition of azides and alkynes has emerged as the frontrunner. TCI offers many chemicals for the Hüisgen 1,3-dipolar [3+2] cycloaddition.

Ligands Accelerating Click Reaction
Copper(I)-catalyzed azide alkyne cycloadditions (CuAAC) are a well-developed and a typical example of so-called "click reaction". Click chemistry has come to high prominence in the past decade due to its applications in chemical biology. Applications have contributed significantly to innovation in the field of chemical biology, in part to "bioorthogonality" of ethynyl and azido groups, which do not interfere with living systems. However, copper(I) anions are easily oxidized and undergo disproportionation, in addition to showing cytotoxic effects. Because of this, the usage of in vivo CuAAC is quite limited. Fortunately, Sharpless and Fokin et al. have recently demonstrated that tertiary amine and triazine bearing ligands can stabilize the copper(I) anion and accelerate reactions. Additionally, water-soluble ligands have also been developed. With these innovations, CuAAC can be conducted on the cell surface or within the cell and can proceed with low loading amount of Cu catalyst, without showing cytotoxicity.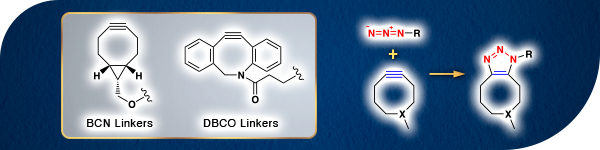
Copper-free Click Reaction Linkers
Generally, the Huisgen cyclization requires some copper salts to promote the reactions. However, use of the copper salt-mediated click reactions for in vivo applications is often highly restricted owing to the toxicity of active oxygen in vivo generated by the copper salt. Therefore, the development of advanced click reactions without using any copper salt has been investigated. In 2004, C. R. Bertozzi et al. reported the copper-free click reaction using highly-strained cyclooctyl groups as reactants. These cyclooctyl compounds are alkyne derivatives performed mainly to improve two chemical properties of second order reactions, rate constant and lipophilicity (log P). They are widely used as a molecular tool to reveal the metabolic systems. For instance, DBCO linkers and BCN linkers are suitable molecules for the copper-free click reaction in biological research because they have low lipophilicity and high reactivity.
TCI Click Chemistry Articles
TCI has many articles focused on specific products among our product line, introducing the product characteristics and means of use. The articles featured on Click Chemistry are following:
» TCI eNewsletter Back IssuesFollow Us on Social Media

|

|

|

|
How to Order TCI ProductsTo order, please contact your local distributor found below.» Our distributors |
| * The prices displayed in the landing website are USD based catalog prices. TCI has specific website and local currency based price list for customers in South Korea, Singapore, and Australia. For customers in other Asia Pacific countries / regions, please visit our Asia Pacific Website and contact our local distributors for the end user's cost. (The USD prices that are shown in Asia Pacific websites are TCI's catalog prices.) Please visit each country/region's website from the following links and confirm the prices. https://www.TCIchemicals.com |
|
If you are interested in our biweekly e-enewsletter "TCI News" and want to receive regularly, please click the link below and sign up. TCI News Sign-Up |
|
Tokyo Chemical Industry Co., Ltd. (TCI) Global Business Department Customer Support and Technical service » globalbusiness@TCIchemicals.com » https://www.TCIchemicals.com |
