TCI Chemistry News
Maximum quantity allowed is 999
Please select the quantity
July 2022
We apologize for the delay in distributing TCI News. This issue covers our chemistry topics as follows.
- Peptide Nucleic Acid (PNA) Monomers
- Organocatalyst for Electrophilic Halogenation of Aromatic Compounds under Mild Conditions
- Palladium-Catalyzed Coupling Reaction of the Heterocyclic Sulfinate
- The Regioselective Koenigs-Knorr-Type Glycosylation of Carbohydrates Using a Borinic Acid Ester Catalyst
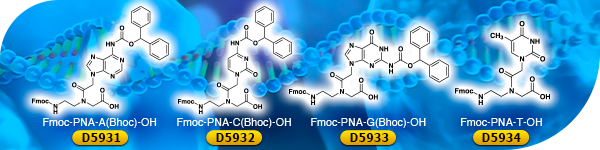
Peptide Nucleic Acid (PNA) Monomers
Peptide nucleic acid (PNA) monomers such as Fmoc-PNA-A(Bhoc)-OH [D5931], Fmoc-PNA-C(Bhoc)-OH [D5932], Fmoc-PNA-G(Bhoc)-OH [D5933] and Fmoc-PNA-T-OH [D5934] are among the most successful synthetic analogues of DNA monomers. For PNA, the sugar-phosphate backbone is replaced by N-(2-aminoethyl)glycine. PNAs feature the stability of hybridization complexes with targeted DNA, the metabolic stability, and the ease of chemical modifications such as fluorescent probe labeling and spacer (linker) insertion. PNAs are applicable to antisense and antigen agents, among other applications.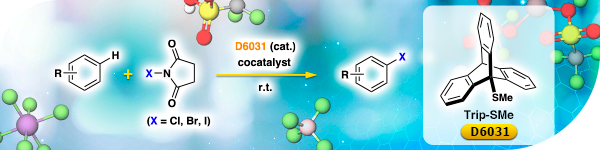
Organocatalyst for Electrophilic Halogenation of Aromatic Compounds under Mild Conditions
Trip-SMe [D6031], a triptycene-based Lewis base catalyst efficiently catalyzes halogenation of various aromatic compounds using N-halosuccinimide and cocatalyst ( AgSbF6, AgBF4, TfOH, In(OTf)3 etc.). The halogenation proceeds regioselectively under mild conditions (room temperature, neutral). Trip-SMe also catalyzes trifluoromethylthiolation of aromatic compounds.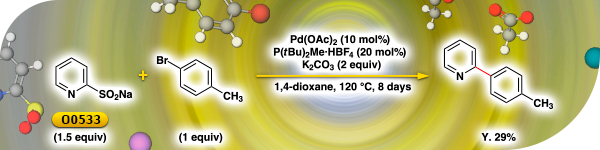
TCI Practical Example: Palladium-Catalyzed Coupling Reaction of the Heterocyclic Sulfinate
The Suzuki-Miyaura cross coupling of pyridine-2-boronic acid with aryl halide does not proceed because pyridine-2-boronic acid is unstable. Pyridine-2-sulfinate can be substituted for pyridine-2-boronic acid. TCI introduces the cross-coupling reaction using sodium pyridine-2-sulfinate [O0533] and 4-bromotoluene by using palladium(II) acetate as a catalyst.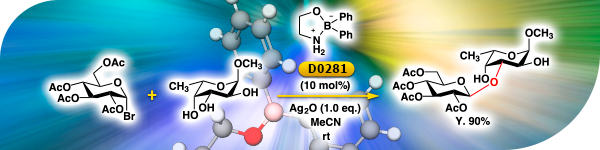
TCI Practical Example: The Regioselective Koenigs-Knorr-Type Glycosylation of Carbohydrates Using a Borinic Acid Ester Catalyst
We are proud to present the regioselective Koenigs-Knorr-type glycosylation of 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide and methyl α-L-fucopyranoside by using 2-aminoethyl diphenylborinate [D0281] as a catalyst. The reaction report is actually performed by TCI's synthesis staff. You can see not only the reaction procedure but also the comment and analysis data from our staff. » TCI eNewsletter Back IssuesFollow Us on Social Media

|

|

|

|
How to Order TCI ProductsTo order, please contact your local distributor found below.» Our distributors |
| * The prices displayed in the landing website are USD based catalog prices. TCI has specific website and local currency based price list for customers in South Korea, Singapore, and Australia. For customers in other Asia Pacific countries / regions, please visit our Asia Pacific Website and contact our local distributors for the end user's cost. (The USD prices that are shown in Asia Pacific websites are TCI's catalog prices.) Please visit each country/region's website from the following links and confirm the prices. https://www.TCIchemicals.com |
|
If you are interested in our biweekly e-enewsletter "TCI News" and want to receive regularly, please click the link below and sign up. TCI News Sign-Up |
|
Tokyo Chemical Industry Co., Ltd. (TCI) Global Business Department Customer Support and Technical service » globalbusiness@TCIchemicals.com » https://www.TCIchemicals.com |
