Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
Please select the quantity
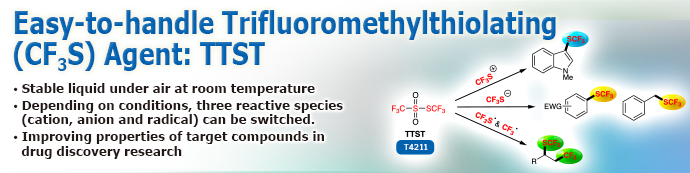
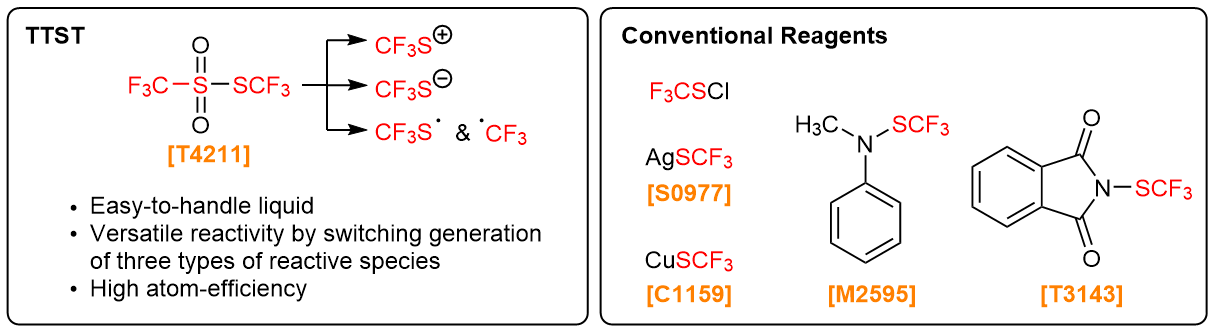
As a trifluoromethylthio (CF3S) group is reported to improve properties of chemicals, it is gathering more attention in pharmaceutical and agricultural chemistry.1,2) Unlike conventional trifluoromethylthiolating agents, TTST contains no metals such as expensive silver. In addition, TTST has no high molecular weight organic parts such as imides. Therefore, TTST does not generates high molecular organic wastes after the reactions.
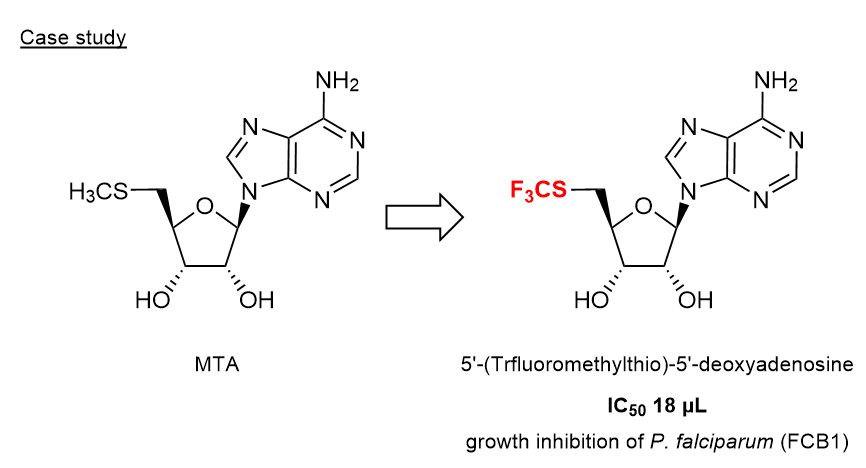
For example, it is reported that replacement of methylthio group in MTA (= 5'-(Methylthio)-5'-deoxyadenosine) to trifluoromethylthio group enhanced growth inhibition of malarial parasite P. falciparum.3)
Advantages
- Liquid that is easy-to-handle in air, at ambient temperature.
- Can switch among generating trifluoromethylthio cation species, anion species, and radical species depending on the reaction conditions.
Applications
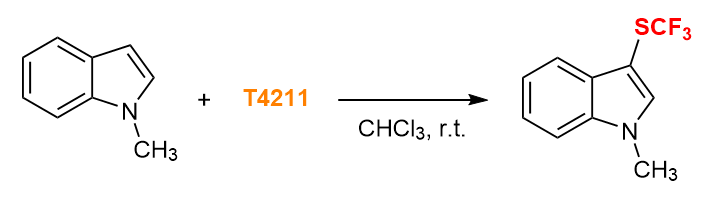
Electrophilic Trifluoromethylthiolation of Electron-rich Aromatic Ring 4)
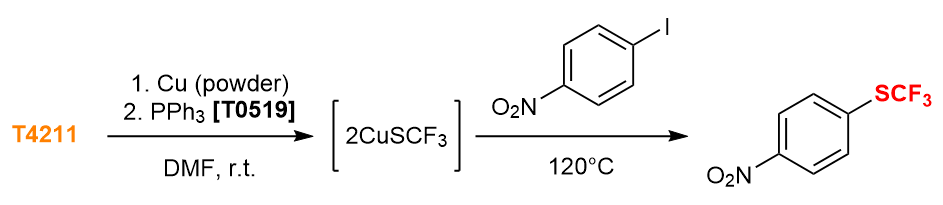
Nucleophilic Trifluoromethylthiolation of Aryl Iodide 4)
Radical Trifluoromethylthiolation of Alkene 4)
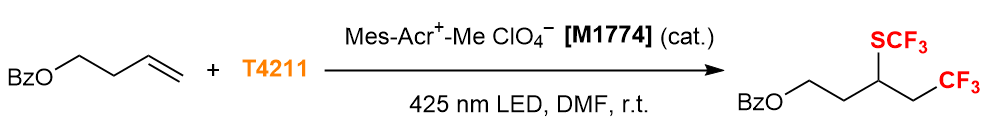
Related Products
Product Brochure
References
- 1) Aromatic substituent constants for structure-activity correlations
- 2) Synthetic Methods for Compounds Having CF3–S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions
- 3) Synthesis and Biological Activity of Fluorinated Intermediate of the Methionine Salvage Pathway
- 4) Preparation and Reactivity Study of a Versatile Trifluoromethylthiolating Agent: S-Trifluoromethyl Trifluoromethanesulfonothioate (TTST)