Maximum quantity allowed is 999
Chiral Ligand for the Synthesis of Optically Active Aziridines
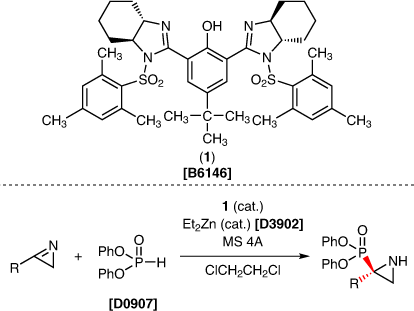
The synthesis of optically active aziridines from 2H-azirines by the addition of alkyl radicals1) and nucleophilic addition of aryl pyrazoles2) has been reported. On the other hand, a reaction using (-)-4-tert-butyl-2,6-bis[(4S,5S)-4,5-tetramethylene-1-(2,4,6-trimethylbenzenesulfonyl)imidazolin-2-yl]phenol (1) as a chiral ligand enables the synthesis of optically active aziridines from 2H-azirines.3) The addition of 2H-azirine and diphenyl phosphite to the reaction mixture of 1, MS 4A, and diethylzinc gives the optically active aziridine. The aziridines can be converted to chiral amines and oxazolines without loss of enantiopurity. Therefore, further applications using 1 are anticipated to be reported in the future.
Related Products
- B6146
- (-)-4-tert-Butyl-2,6-bis[(4S,5S)-4,5-tetramethylene-1-(2,4,6-trimethylbenzenesulfonyl)imidazolin-2-yl]phenol
- D0907
- Diphenyl Phosphite
- D3902
- Diethylzinc (ca. 15% in Toluene, ca. 1mol/L)
References
- 1) Lewis acid-catalyzed asymmetric radical additions of trialkylboranes to (1R,2S,5R)-2-(1-methyl-1-phenylethyl)-5-methylcyclohexyl-2H-azirine-3-carboxylate
- 2) Organocatalyzed nucleophilic addition of pyrazoles to 2H-azirines: asymmetric synthesis of 3,3-disubstituted aziridines and kinetic resolution of racemic 2H-azirines
- 3) Enantioselective Reaction of 2H-Azirines with Phosphite Using Chiral Bis(imidazoline)/Zinc(II) Catalysts