Maximum quantity allowed is 999
Please select the quantity
CAS RN: 15956-28-2 | Product Number: R0069
Rhodium(II) Acetate Dimer

Purity:
Synonyms:
- Dirhodium(II) Tetraacetate
- Tetrakis(acetato)dirhodium(II)
Product Documents:
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | R0069 |
| Molecular Formula / Molecular Weight | C__8H__1__2O__8Rh__2 = 441.99 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Packaging and Container | 100MG-Glass Bottle with Plastic Insert (View image), 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 15956-28-2 |
| Reaxys Registry Number | 14201302 |
| PubChem Substance ID | 87575627 |
| MDL Number | MFCD00003538 |
Specifications
| Appearance | Green to Dark green powder to crystal |
| Elemental analysis(Carbon) | 20.50 to 22.70 % |
Properties (reference)
| Melting Point | 240 °C |
GHS
Related Laws:
| RTECS# | VI9361000 |
Transport Information:
| H.S.code* | 2843.90-000 |
Application
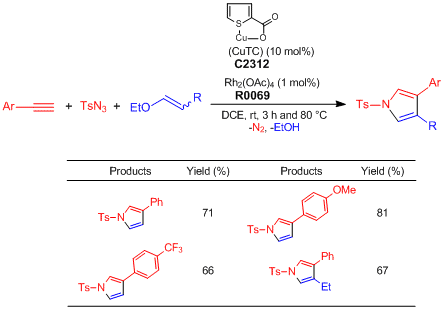
One-pot Synthesis of Pyrroles from Terminal Alkynes, N-Sulfonyl Azides, and Alkenyl Alkyl Ethers
Typical Procedure:
Alkyne (0.2 mmol), TsN3 (1.0 eq.), alkenyl alkyl ether (3.0 eq.), CuTC (10.0 mol%), Rh2(OAc)4 (1.0 mol%), and DCE (0.2 M) are added to an oven-dried test tube under nitrogen atmosphere. The reaction mixture is stirred at ambient temperature for 3 h and then, heated at 80 °C for 4–13 h. After the reaction mixture is filtered through short path of celite with CH2Cl2, the filtrate is concentrated under reduced pressure. The residue is purified by silica gel column chromatography (ethyl acetate–hexane) to give pyrrole derivatives (Y. 65–81%).
Alkyne (0.2 mmol), TsN3 (1.0 eq.), alkenyl alkyl ether (3.0 eq.), CuTC (10.0 mol%), Rh2(OAc)4 (1.0 mol%), and DCE (0.2 M) are added to an oven-dried test tube under nitrogen atmosphere. The reaction mixture is stirred at ambient temperature for 3 h and then, heated at 80 °C for 4–13 h. After the reaction mixture is filtered through short path of celite with CH2Cl2, the filtrate is concentrated under reduced pressure. The residue is purified by silica gel column chromatography (ethyl acetate–hexane) to give pyrrole derivatives (Y. 65–81%).
References
Application
Highly Diastereoselective Synthesis of Fully Substituted Tetrahydrofurans

Typical Procedure: A flask is charged with allyl alcohol (0.10 mmol, 1.0 eq.), Rh2(OAc)4 (2.0 mol%) and 4A MS (0.1 g) in toluene (1.5 mL), the reaction mixture is stirred at 45℃, and a solution of the diazo compound (0.20 mmol) in toluene (0.5 mL) is added to the reaction mixture over a period of 1 h through a syringe pump. After completion of the addition, piperidine (2.0 eq.) is added and the reaction mixture is stirred for additional 5-10 h (reaction completion is monitored by TLC) at 45℃. The reaction mixture is purified by flash chromatography on silica gel (EtOAc : light petroleum ether = 1 : 50 to 1 : 20) to give the pure product.
References
PubMed Literature
Articles/Brochures
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




