Maximum quantity allowed is 999
请选择数量
CAS RN: 77-48-5 | 產品號碼: D1265
1,3-Dibromo-5,5-dimethylhydantoin

* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | D1265 |
| 純度/分析方法 | >97.0%(T) |
| 分子式 / 分子量 | C__5H__6Br__2N__2O__2 = 285.92 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 儲存在惰性氣體下 | Store under inert gas |
| 應避免的情況 | Moisture Sensitive |
| CAS RN | 77-48-5 |
| Reaxys-RN | 146024 |
| PubChem Substance ID | 87567800 |
| SDBS (AIST Spectral DB) | 4013 |
| Merck Index(14) | 3017 |
| MDL編號 | MFCD00003189 |
產品規格
| Appearance | White to Light yellow to Light orange powder to crystal |
| Purity(Iodometric Titration) | min. 97.0 % |
| Solubility in hot Toluene | almost transparency |
性質
| 熔點 | 193 °C |
| 沸點 | 376 °C |
| 溶解性(可溶於) | Chloroform, Ethanol |
GHS
| 圖形表示 |




|
| 信號詞 | Danger |
| 危險性說明 | H301 : Toxic if swallowed. H314 : Causes severe skin burns and eye damage. H410 : Very toxic to aquatic life with long lasting effects. H272 : May intensify fire; oxidizer. |
| 防範說明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P273 : Avoid release to the environment. P260 : Do not breathe dusts or mists. P270 : Do not eat, drink or smoke when using this product. P220 : Keep/Store away from clothing/ combustible materials. P210 : Keep away from heat. P221 : Take any precaution to avoid mixing with combustibles. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. P391 : Collect spillage. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P301 + P310 + P330 : IF SWALLOWED: Immediately call a POISON CENTER/doctor. Rinse mouth. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P405 : Store locked up. |
相關法規
| RTECS # | MU0686000 |
運輸資料
| UN編號 | UN3087 |
| 類別 | 5.1 / 6.1 |
| 包裝類別 | II |
| HS編碼* | 2933.21-000 |
Application
Carbon-Heteroatom Bond Formation at the Benzylic Position of Methylarenes

Typical procedure (Ar = 4-BrPh, nucleophile = PhCO2H): To a solution of 4-bromotoluene (342.1 mg, 2.0 mmol) in anhydrous MeCN (4 mL) are added 1,3-dibromo-5,5-dimethylhydantoin (314.5 mg, 1.1 mmol) and AIBN (16.8 mg, 0.1 mmol) at room temperature. The mixture is stirred for 2 h at 90 °C. Then, benzoic acid (268.7 mg, 2.2 mmol) and DIPEA (0.35 mL, 2.0 mmol) are added at room temperature and the mixture is stirred for 16 h at 90 °C. The mixture is quenched by the addition of saturated aq. Na2SO3 solution (10 mL) and then extracted with CHCl3 (3 × 20 mL). The organic layer is dried over Na2SO4. After removal of the solvent under reduced pressure, the residue is purified by column chromatography on silica gel (eluent: hexane/CHCl3 = 1/1) to afford 4-bromobenzyl benzoate as a white solid. (489.1 mg, 84% yield).
References
Application
Selective ortho-Monobromination of Phenols and Polyphenols
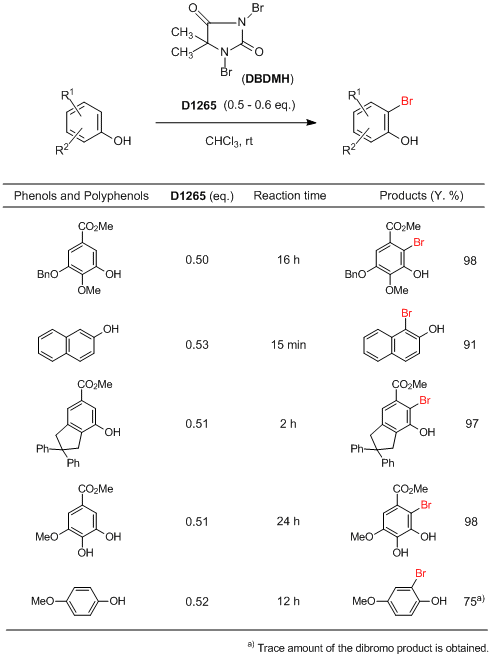
Typical Procedure:
DBDMH (0.50–0.53 eq.) is added in part into the solution of starting material in CHCl3 (5–7 mL / mmol) at room temperature. Upon addition of the DBDMH, the solution becomes red or deep brown colored, the next portion of DBDMH is added after the disappearance of color and so on. The progress of the reaction is monitored by GC-MS. After completion of the reaction, removal of the solvent followed by the separation of the solid byproduct (derived from DBDMH) by simple filtration provides the almost pure bromide. 10% Aqueous sodium hydrosulfite solution is added into the reaction mixture, stirred for 5 min. Organic layer is separated, dried over MgSO4 and concentrated in evaporator yields the almost pure product. Passing through a small chromatographic column, it gives the pure products.
DBDMH (0.50–0.53 eq.) is added in part into the solution of starting material in CHCl3 (5–7 mL / mmol) at room temperature. Upon addition of the DBDMH, the solution becomes red or deep brown colored, the next portion of DBDMH is added after the disappearance of color and so on. The progress of the reaction is monitored by GC-MS. After completion of the reaction, removal of the solvent followed by the separation of the solid byproduct (derived from DBDMH) by simple filtration provides the almost pure bromide. 10% Aqueous sodium hydrosulfite solution is added into the reaction mixture, stirred for 5 min. Organic layer is separated, dried over MgSO4 and concentrated in evaporator yields the almost pure product. Passing through a small chromatographic column, it gives the pure products.
References
- 1,3-Dibromo-5,5-dimethylhydantoin, a useful reagent for ortho-monobromination of phenols and polyphenols
- A. Alam, Y. Takaguchi, S. Tsuboi, J. Fac. Environ. Sci. Tech., Okayama Univ. 2005, 10, 105.
考研文獻
文章/手冊
TCIMail
[Research Articles] Carbon-Heteroatom Bond Formation at the Benzylic Position of Methylarenes產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜
請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。




