Maximum quantity allowed is 999
Please select the quantity
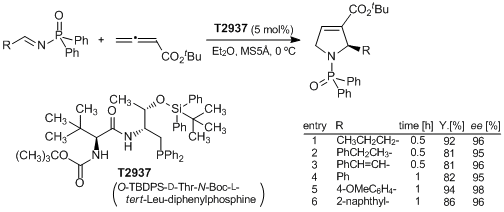
A highly Enantioselective [3+2] Cyclization between Imines and Allenoates Catalyzed by a Dipeptide-Based Chiral Phosphine
Lu et al. have reported a highly enantioselective [3+2] cyclization between imines and allenoates using a dipeptide-base chiral phosphine (O-TBDPS-D-Thr-N-Boc-L-tert-Leu-diphenylphosphine) as a catalyst. The reaction proceeds in the presence of 5 mol% of the catalyst, and is complete within an hour. The 2-alkyl- or 2-aryl-substituted 3-pyrroline products are obtained in good yield and with high enantioselectivities. They demonstrate the synthesis of the pyrrolizidine alkaloid (+)-trachelanthamidine using the catalyst as a key step.