Notice of System Recovery: System maintenance has been completed. We deeply apologize for the inconvenience caused by exceeding the scheduled completion time.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News July 2025 | [Product Highlights] Travoprost with Intra-Ocular-... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
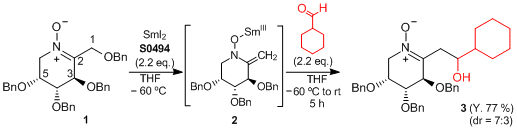
Tandem SmI2-induced β-elimination / aldol-type reaction
Racine and Py have reported a novel tandem SmI2-induced β-elimination / aldol-type reaction. Upon treatment with SmI2 (0.1 mol/L in THF), a nitrone, bearing a benzyloxy group at the β position, underwent the beta-elimination of the benzyloxy group. The intermediate was reacted with cyclohexanecarboxaldehyde as an electrophile to produce an aldol-type adduct. The tandem process results in the transformation of a C–O bond into a C–C bond.