Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [Product Highlights] Cell Culture-Ready Antibiotic... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 819050-89-0 | Product Number: B4549
Bis[rhodium(α,α,α',α'-tetramethyl-1,3-benzenedipropionic Acid)]
Purity: >96.0%(HPLC)
Synonyms:
- Rh2(esp)2
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 100MG |
$91.00
|
≥60 | ≥100 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | B4549 |
Purity / Analysis Method 
|
>96.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__3__2H__4__0O__8Rh__2 = 758.48 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
Packaging and Container 
|
100MG-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 819050-89-0 |
| Reaxys Registry Number | 21493662 |
| PubChem Substance ID | 354333338 |
| MDL Number | MFCD08457636 |
Specifications
| Appearance | Yellow to Brown to Dark green powder to crystal |
| Purity(HPLC) | min. 96.0 area% |
| NMR | confirm to structure |
Properties (reference)
GHS
Related Laws:
Transport Information:
| H.S.code* | 2843.90-000 |
Application
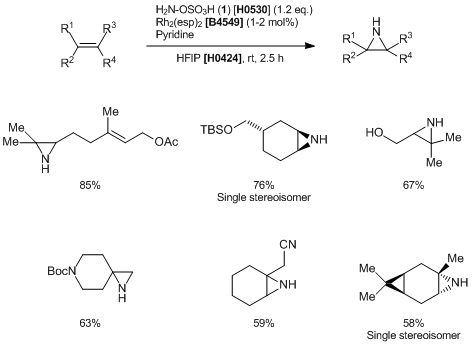
Synthesis of N-H Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acid

Reference
- Direct and Stereospecific Synthesis of N-H and N-Alkyl Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acids
Application
Synthesis of 1,2-Diamine Derivatives Using a Rhodium Catalyst
References
- Direct and Stereospecific Synthesis of N-H and N-Alkyl Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acids
Application
Direct Synthesis of N-Unprotected Aziridines from Olefins Using a Rhodium Catalyst

Representative procedure: A round bottom flask is charged with alkene (0.5 mmol, 1.0 eq.) and CF3CH2OH (5 mL). To this solution at the specified temperature are added Rh2(esp)2 (3.8 mg, 5 µmol, 1 mol%) and 1 (0.119 g, 0.6 mmol, 1.2 eq.). The reaction is stirred at the specified temperature and monitored by TLC. More catalyst and 1 are added, if required. After completion, the reaction mixture is diluted with CH2Cl2(10 mL) and washed once with 15% aq. NaHCO3 solution (5 mL). The aqueous layer is extracted twice with CH2Cl2 (10 mL) and the combined organic portions are washed once with brine (5 mL), dried over Na2SO4, and concentrated in vacuo. The residue is purified on pre-packed SiO2 column.
References
- Direct Stereospecific Synthesis of Unprotected N-H and N-Me Aziridines from Olefins
PubMed Literature
Documents
Product Articles
[Product Highlights] Synthesis of N-H Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acid[Research Articles] Synthesis of 1,2-Diamine Derivatives Using a Rhodium Catalyst
[Research Articles] Direct Synthesis of N-Unprotected Aziridines from Olefins Using a Rhodium Catalyst
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.
![Bis[rhodium(alpha,alpha,alpha',alpha'-tetramethyl-1,3-benzenedipropionic Acid)] Thumbnail of Bis[rhodium(alpha,alpha,alpha',alpha'-tetramethyl-1,3-benzenedipropionic Acid)]](/medias/B4549.jpg?context=bWFzdGVyfHJvb3R8MTk0MTd8aW1hZ2UvanBlZ3xhRGxqTDJnNE5pODRPVE16TnpJek5UTTNORE00TDBJME5UUTVMbXB3Wnd8ODVkYmNmM2QxOTFiMzIyYTlmNjM5NTI5OTEwMjYxMmFiZmMyYThlMGRjZTNiYzUyYjE1MGYyMmFjZDdlYzE3Yg)
![Bis[rhodium(alpha,alpha,alpha',alpha'-tetramethyl-1,3-benzenedipropionic Acid)] Chemical Structure of Bis[rhodium(alpha,alpha,alpha',alpha'-tetramethyl-1,3-benzenedipropionic Acid)]](/medias/B4549.jpg?context=bWFzdGVyfHJvb3R8NzUyOTF8aW1hZ2UvanBlZ3xhR1UyTDJoa015ODRPVEl3TWpjMk56azFOREl5TDBJME5UUTVMbXB3Wnd8MDA3OGYyNzExYzcwNzU1NTI4MDliYWU5ZmIxOTdmNzUyMDY1OTA4YWM4MTdkMjZhNGM2ZTcwOGUxYTVhNDE3Yg)
![Bis[rhodium(α,α,α',α'-tetramethyl-1,3-benzenedipropionic Acid)] Bis[rhodium(α,α,α',α'-tetramethyl-1,3-benzenedipropionic Acid)]](/_ui/responsive/theme-tci/images/missing_product_zoom.webp)

