Maximum quantity allowed is 999
请选择数量
Efficient Bis(oxazolidine)pyridine Ligands Utilized for the Asymmetric [3+2] Cycloaddition
No.161(April 2014)
- B4227
- 2,6-Bis[(2S,4S)-4-methyl-5,5-diphenyloxazolidin-2-yl]pyridine (1) (*This product is discontinued.)
- B4228
- 2,6-Bis[(2S,5S)-4,4-diphenyl-1-aza-3-oxabicyclo[3.3.0]octan-2-yl]pyridine (2)
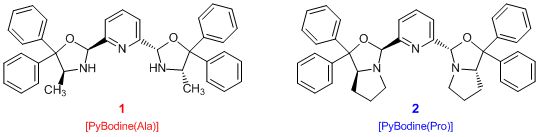
Bis(oxazolidine)pyridine (=PyBodine) ligands are tridentate ligands developed by Arai et
al. PyBodine ligands 1 and 2 are easily prepared from optically active hydroxyamines derived
from the related amino acids allowing the design of various chiral asymmetrical fields by replacing optically
active hydroxyamines. The reactivity and enantioselectivity of various PyBodine ligands are evaluated by using a
high-throughput screening system for analyzing the asymmetric induction with circular dichroism as a detector. As
a result, in the presence of copper(II) acetate, the ligands 1 and 2 derived from L-alanine and
L-proline respectively, show high reactivities for the catalytic asymmetric [3+2] cycloaddition of azomethine
imines with propiolates. In this reaction, when using 1 as a ligand, (R)-adducts of
N,N-bicyclic pyrazolidinone derivatives are given with excellent enantioselectively. Whereas,
(S)-adducts are enantioselectively formed by using the ligand 2. Thus, the ligands 1 and
2 can be complementarily used for the enantioselective synthesis of R/S-isomers.

References
- Development of a tailor-made bis(oxazolidine)pyridine–metal catalyst for the [3+2] cycloaddition of azomethine imines with propiolates
The prices are subject to change without notice. Please confirm the newest price by our online
catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.