Maximum quantity allowed is 999
请选择数量
Oxime-Derived Palladacycles as Versatile Catalysts in Cross-Coupling Reactions
No.146(August 2011)
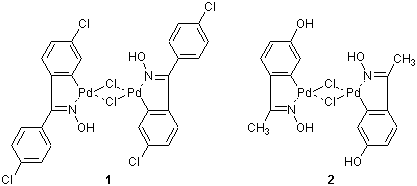
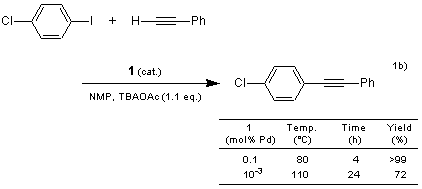
Recently, metallacycles, which are derivatives of carbocyclic compounds wherein a metal has replaced at least one carbon center, have attracted attention for their high catalytic reactivity. Palladacyles 1 and 2, developed by Nájera et al., are among such metallacycles, and have been used as catalysts for carbon-carbon bond formation. They are thermally stable and not sensitive to air or moisture.1a) According to their result, 1 and 2 exhibit high catalytic activity in organic solvents as well as in aqueous solvents. For example, in the Sonogashira-type coupling reaction using 1, the desired acetylenes are obtained in good yields with no addition of copper and amines.1b) On the other hand, in the Hiyama-type coupling reaction using 2, the desired styrene derivatives are obtained without using fluorides.1c)

Furthermore, there are also several reports on applications to other cross-coupling reactions, such as the Suzuki-Miyaura1d) and Heck1e) reactions.
References
- 1)Synthetic applications of oxime-derived palladacycles as versatile catalysts in cross-coupling reactions
- a) D. A. Alonso, L. Botella, C. Nájera, M. C. Pacheco, Synthesis 2004, 1713.

- b) D. A. Alonso, C. Nájera, M. C. Pacheco, Tetrahedron Lett. 2002, 43, 9365.

- c) E. Alacid, C. Nájera, Adv. Synth Catal. 2006, 348, 2085.

- d) D. A. Alonso, C. Nájera, M. C. Pacheco, J. Org. Chem. 2002, 67, 5588.

- e) L. Botella, C. Nájera, Tetrahedron 2004, 60 5563; L. Botella, C. Nájera, Tetrahedron Lett. 2004, 45, 1833.

- a) D. A. Alonso, L. Botella, C. Nájera, M. C. Pacheco, Synthesis 2004, 1713.
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.