Maximum quantity allowed is 999
请选择数量
A Catalyst for Asymmetric Baylis-Hillman Reaction
No.146(August 2011)

A cinchona alkaloid, β-isocupreidine (1), is an effective catalyst in the asymmetric Baylis-Hillman reaction. The reaction with aldehydes and 1,1,1,3,3,3-hexafluoroisopropyl acrylate (HFIPA) by the use of the catalyst 1 (0.1 eq.) gives the corresponding adducts with high enantioselectivity.1)

β-Isocupreidine (1) also catalyzes the asymmetric aza-Baylis-Hillman reaction.2)
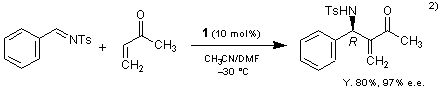
Typical Procedure: Synthesis of 21)
To the solution of benzaldehyde (1.0 mmol) and β-isocupreidine (1, 0.1 mmol) in DMF (1 mL) at −55 °C was added HFIPA (1.3 mmol). After stirring at −55 °C for 48 h, the reaction was quenched with 0.1 M HCl (3 mL). The reaction mixture was extracted with EtOAc, washed with saturated NaHCO3 and brine, dried over MgSO4, concentrated, and chromatographed on silica gel (hexane-EtOAc) to give 2 (Y. 75%, 97% ee).
To the solution of benzaldehyde (1.0 mmol) and β-isocupreidine (1, 0.1 mmol) in DMF (1 mL) at −55 °C was added HFIPA (1.3 mmol). After stirring at −55 °C for 48 h, the reaction was quenched with 0.1 M HCl (3 mL). The reaction mixture was extracted with EtOAc, washed with saturated NaHCO3 and brine, dried over MgSO4, concentrated, and chromatographed on silica gel (hexane-EtOAc) to give 2 (Y. 75%, 97% ee).
* * *
It was reported that the reaction yield would decrease by using 1 that absorbed moisture in the air. We have taken all measures to avoid moisture during manufacturing and shipping. If the reaction result is not desirable, a good result may be obtained after dissolving 1 into tetrahydrofuran followed by azeotropic removal of water under reduced pressure.
It was reported that the reaction yield would decrease by using 1 that absorbed moisture in the air. We have taken all measures to avoid moisture during manufacturing and shipping. If the reaction result is not desirable, a good result may be obtained after dissolving 1 into tetrahydrofuran followed by azeotropic removal of water under reduced pressure.
References
- 1)β-Isocupreidine-hexafluoroisopropyl acrylate method for asymmetric Baylis-Hillman reaction
- 2)Catalytic, asymmetric aza-Baylis-Hillman reaction of N-sulfonated imines with activated olefins by quinidine-derived chiral amines
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.