Maximum quantity allowed is 999
请选择数量
Phosphorylation
No.104(October 1999)
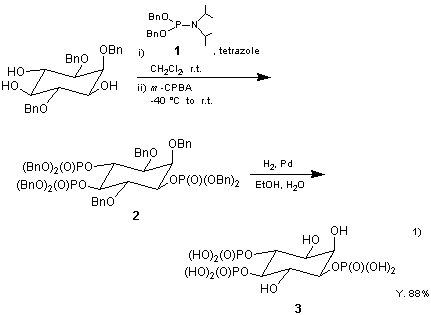
The present reagent 1 reacts rapidly with alcohols in the presence of 1H-tetrazole at room temperature to generate phosphorous triesters. Subsequent reaction with oxidizing agents such as m-chloroperoxybenzoic acid affords the corresponding phosphoric triesters 2. The triesters thus obtained can be deprotected by cleavage of the benzyl group by catalytic hydrogenolysis using Pd/C to yield the desired derivatives of phosphoric acids 31). The reagent 1 has high reactivity and its reactions to proceed under mild conditions. It has a wide scope of applications, including the synthesis of biomolecules such as oligonucleotides2).
References
- 1) Phosphorylation of cyclitols
- 2) The synthesis of oligonucleotides
- 3) Other applications
- a) Phosphorylation of amino acids
- b) Phosphorylation of peptides
- c) Phosphorylation of sugars
Related Compounds
- C0978
- Barium 2-Cyanoethylphosphate
- C1210
- 2-Chloro-4H-1,3,2-benzodioxaphosphorin-4-one
- C1215
- 2-Chloro-1,3,2-dioxaphospholane
- C1250
- 2-Chloro-2-oxo-1,3,2-dioxaphospholane
- C1018
- 2-Chlorophenyl-N-phenylphosphoramidochloridate
- C1019
- 4-Chlorophenyl-N-phenylphosphoramidochloridate
- C0976
- 2-Chlorophenylphosphorodichloridate
- C0977
- 4-Chlorophenylphosphorodichloridate
- D1613
- S,S'-Diphenylphosphorodithioate Monocyclohexylammonium Salt
- D1059
- Diphenylphosphoryl Chloride
- M0904
- Methyl Phosphorodichloridate
- M0905
- Methyl Phosphorodichloridite
- P0209
- Phenylphosphoryl Dichloride
- P1223
- Pyrophosphoric Acid Tetrabenzyl Ester
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.