Maximum quantity allowed is 999
TCI Practical Example: Construction of the Terminal Alkyne Using Ohira-Bestmann Reagent
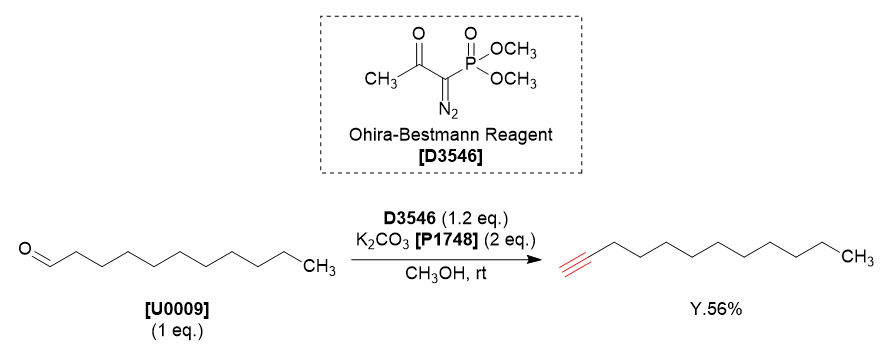
We are proud to introduce the synthesis of 1-dodecyne utilizing Ohira-Bestmann reagent and undecanal.
-
Used Chemicals
-
Procedure
-
Potassium carbonate (0.32 g,2.4 mmol) and Ohira-Bestmann reagent (0.27 g, 1.4 mmol) were added to a methanol (10 mL) solution of undecanal (0.20 g, 1.2 mmol) at room temperature under nitrogen atmosphere. The reaction mixture was stirred overnight at room temperature, then diluted with diethyl ether and washed with saturated aqueous sodium bicarbonate, and dried by sodium sulfate. The organic layer was concentrated under reduced pressure. The resulting crude product was purified by column chromatography (hexane:toluene = 3:1 on silica gel) to give 1-dodecyne as a colorless liquid (0.11 g, 56% yield).
-
Experimenter’s Comments
-
The reaction mixtures were monitored by 1H NMR (CDCl3).
-
Analytical Data
-
1-Dodecyne
1H NMR (400 MHz, CDCl3); δ 2.18 (t, J = 7.1 Hz, 2H), 1.94 (s, 1H), 1.57-1.46 (m, 2H), 1.44-1.33 (m, 2H), 1.33-1.19 (m, 12H), 0.88 (t, J = 6.4 Hz, 3H).
13C NMR (101 MHz, CDCl3); δ 85.0, 68.2, 32.0, 29.7, 29.7, 29.5, 29.3, 28.9, 28.6, 22.8, 18.5, 14.3.
-
Lead Reference
-
- Further Improvements of the Synthesis of Alkynes from Aldehydes
-
Other Reference
-
- Methanolysis of Dimethyl (1-Diazo-2-oxopropyl) Phosphonate: Generation of Dimethyl (Diazomethyl) Phosphonate and Reaction with Carbonyl Compounds