Maintenance Notice (3:00 AM - 8:30 AM November 1, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News October 2025 | [Product Highlights] Tetraphenylethylene Derivatives Used for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
Cross-linkers Containing Photoreactive Diazirine Group
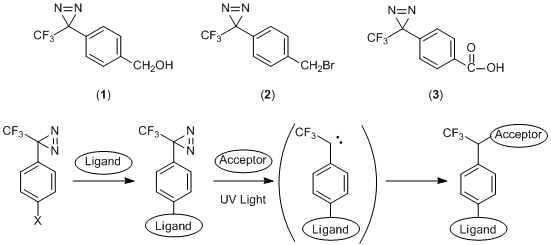
Diazirine changes to a high-reactive carbene by absorbing light near 360 nm, which forms a covalent bond to a nearby molecule. The covalent bond is more stable than one formed by nitrene derived from a photoreactive azide1). Phenyldiazirine derivatives, 4-[3-(Trifluoromethyl)-3H-diazirin-3-yl]benzyl Alcohol (1), 4-[3-(Trifluoromethyl)-3H-diazirin-3-yl]benzyl Bromide (2), 4-[3-(Trifluoromethyl)-3H-diazirin-3-yl]benzoic Acid (3) have a functional group at the para position. For example, after the functional group attaches to a ligand, the diazirine group reacts with the acceptor molecule under photoexcitation. It has been reported in research of the photoaffinity labelings which include the crosslinker with peptides or sugars2), and the photoaffinity microarrays3).
References
- 1)Reviews
- 2)Photoaffinity labeling
- a)Y. Kashiwayama, T. Tomohiro, K. Narita, M. Suzumura, T. Glumoff, J. K. Hiltunen, P. P. V. Veldhoven, Y. Hatanaka, T. Imanaka, J. Biol. Chem. 2010, 285, 26315.

- b)E. W. S. Chan, S. Chattopadhaya, R. C. Panicker, X. Huang, S. Q. Yao, J. Am. Chem. Soc. 2004, 126, 14435.

- c)K. Matsuda, M. Ihara, K. Nishimura, D. B. Sattelle, K. Komai, Biosci. Biotechnol. Biochem. 2001, 65, 1534.

- d)M. Wiegand, T. K. Lindhorst, Eur. J. Org. Chem. 2006, 4841.

- a)Y. Kashiwayama, T. Tomohiro, K. Narita, M. Suzumura, T. Glumoff, J. K. Hiltunen, P. P. V. Veldhoven, Y. Hatanaka, T. Imanaka, J. Biol. Chem. 2010, 285, 26315.
- 3)Photoaffinity microarray
- 4)Phenyldiazirine Synthesis