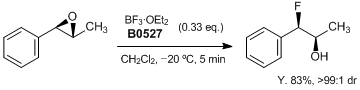Maximum quantity allowed is 999
请选择数量
Stereoselective Ring-Opening Hydrofluorination of Aryl Epoxides
Davies et al. have reported a stereoselective ring-opening hydrofluorination of aryl epoxides with 0.33 equiv of BF3•OEt2 at 20 °C to give rapidly the corresponding syn-fluorohydrins (> 99:1 dr). Mechanistic evidence suggested that a stereoselective SN1-type epoxide ring-opening process involving coordination of BF3 to the oxirane oxygen and transferring of fluorine.