Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
Aza-Cope Rearrangement
The aza-Cope rearrangement is one of the [3,3]-sigmatropic rearrangement reactions of nitrogen-substituted 1,5-hexadienes. The reaction mainly proceeds via a chair-like transition state as in the Cope rearrangement. The rearrangement can proceed regardless of the position of the nitrogen atom, and the 2-aza-Cope rearrangement is often utilized. The 3-aza-Cope rearrangement is the same as the aza-Claisen rearrangement and the 1-aza-Cope rearrangement is regarded as the retro-reaction of the aza-Claisen rearrangement. The aza-Cope rearrangement can be applied to the formation of seven-membered rings like the Cope rearrangement and many studies have been reported of the tandem type aza-Cope/Mannich reaction. Thanks to these reactions, heterocyclic compounds with fused-ring structures can be easily formed.
- Reagents:
- Acids
- Reactants:
- N-Substituted-1,5-hexadienes
- Products:
- N-Substituted-1,5-hexadienes
- Scheme:
-
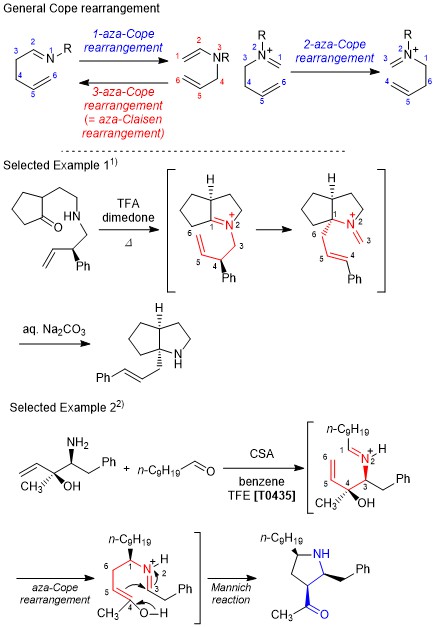
- Original literature:
-
- A Cleavage Reaction of α-Allylbenzylamines
- Review literature:
-
- The 1-aza-Cope rearrangement
-
- The Aza-Cope/Mannich Reaction
Related Name Eeactions
- Cope Rearrangement
- Oxy-Cope Rearrangement
- Claisen Rearrangement
- Mannich Reaction