Maximum quantity allowed is 999
CAS RN: 13675-18-8 | 產品號碼: T2953
Tetrahydroxydiboron
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| Appearance | White to Almost white powder to crystal |
| Purity(Neutralization titration) | 98.0 to 110.0 % |
| 熔點 | 290 °C |
| 溶解性(可溶於) | Methanol |
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防範說明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| HS編碼* | 2810.00-000 |

-
Used Chemicals
-
Procedure
-
To a solution of trans-4-nitrostilbene (1.13 g, 5.00 mmol), diboronic acid (1.34 g, 15.0 mmol, 3.0 eq.) in DMF (30 mL) was added 4,4’-bipyridyl (3.9 mg, 0.025 mmol, 0.50 mol%) at r.t. and the mixture was stirred at the same temperature for 1 hour. The reaction mixture was diluted by 1 mol/L NaHCO3 aq. (100 mL) and the resulting solid was filtered off. The solid was purified by column chromatography (dichloromethane:hexane = 0:1 - 4:1 on silica gel) to give trans-4-aminostilbene as a yellow solid (891 mg, y. 91%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by UPLC.
-
Analytical Data
-
trans-4-Aminostilbene
1H NMR (270 MHz, CDCl3); δ 7.47 (d, 2H, J = 7.0 Hz), 7.38-7.30 (m, 4H), 7.21 (t, 1H, J = 7.0 Hz), 7.03 (d, 1H, J = 15 Hz), 6.91 (d, 1H, J = 15 Hz), 6.63 (d, 2H, J = 8.6 Hz), 3.75 (brs, 2H).
-
Lead Reference
-
- Metal-Free, Rapid, and Highly Chemoselective Reduction of Aromatic Nitro Compounds at Room Temperature
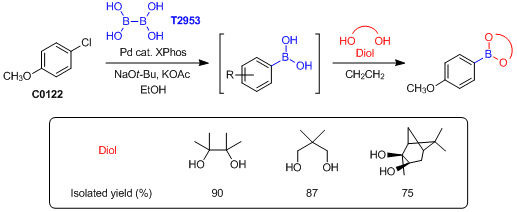
To an oven-dried glass vessel is added (X-Phos) palladium(II) phenethylamine chloride (7.38 mg, 0.01 mmol), X-Phos (9.52 mg, 0.02 mmol), tetrahydroxydiborane, (133.5 mg, 1.5 mmol), KOAc (294 mg, 3 mmol), and NaOt-Bu (1 mg, 0.01 mmol). The vessel is evacuated and backfilled with N2 (three times). EtOH (10 mL degassed) is added followed by the addition of 4-chloroanisole (1 mmol). The reaction is then heated to 80 °C for 18 hours. The reaction is cooled to rt then filtered through a thin pad of Celite (eluting with EtOAc) and concentrated. To the residue is added 1 M aqueous HCl and EtOAc (25 mL each). This mixture is stirred 30 min and the aqueous layer is removed, and the organic layer is washed with brine. The combined aqueous layers are extracted with EtOAc (3 x 10 mL). The combined organics are dried (Na2SO4) and then concentrated. The crude mixture is taken up in CH2Cl2, the corresponding diol is added (1.35 mmol), and the crude reaction is allowed to stir at rt. At completion of the reaction, the reaction is concentrated, and the residue is purified by flash column chromatography to afford the desired aryl boric acid derivatives.
References
- 1)Palladium-Catalyzed, Direct Boronic Acid Synthesis from Aryl Chlorides: A Simplified Route to Diverse Boronate Ester Derivatives
[Product Highlights] Diboronic Acid Usable for the Direct Synthesis of Aromatic Boric Acid Derivatives
SDS
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
示例 CoA
目前沒有該產品的 CoA 示例。
分析圖譜

很抱歉,您搜索的分析圖譜無法提供。





