Maximum quantity allowed is 999
CAS RN: 131274-22-1 | 產品號碼: T2584
Tri-tert-butylphosphonium Tetrafluoroborate
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | T2584 |
| 純度/分析方法 | >98.0%(T) |
| 分子式 / 分子量 | C__1__2H__2__8BF__4P = 290.13 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 包裝和容器 | 1G-Glass Bottle with Plastic Insert (閲覽圖片) |
| CAS RN | 131274-22-1 |
| Reaxys-RN | 8813613 |
| PubChem Substance ID | 125307337 |
| MDL編號 | MFCD04039975 |
| Appearance | White to Almost white powder to crystal |
| Purity(Iodometric Titration) | min. 98.0 % |
| NMR | confirm to structure |
| 熔點 | 261 °C |
| 圖形表示 |


|
| 信號詞 | Danger |
| 危險性說明 | H302 : Harmful if swallowed. H314 : Causes severe skin burns and eye damage. |
| 防範說明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dust. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P405 : Store locked up. |
| UN編號 | UN1759 |
| 類別 | 8 |
| 包裝類別 | III |
| HS編碼* | 2931.49-900 |
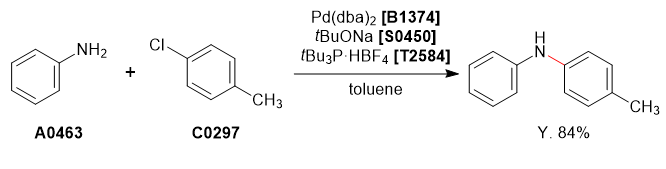
Used Chemicals
Procedure
Aniline (1.0 g, 10.7 mmol) and 4-chlorotoluene (1.4 g, 11.8 mmol) were dissolved in degassed toluene (10 mL) under nitrogen atmosphere. To this solution was added sodium tert-butoxide (1.54 g, 16.1 mmol), tri-tert-butylphosphonium tetrafluoroborate (31.1 mg, 0.1 mmol) and Pd(dba)2 (61.7 mg, 0.1 mmol, 1.5mol%). The reaction mixture was refluxed for 24 h and cooled to room temperature and quenched with H2O (10 mL). The organic phase was separated and washed with brine (10 mL), dried over MgSO4 (10 g), concentrated under reduced pressure to afford the crude product as a brown liquid. The crude product was purified by column chromatography on silica-gel (hexane : ethyl acetate = 95 : 5) to afford the desired product as a pale yellow crystalline solid (1.21 g, 6.6 mmol, 62%).
Experimenter’s Comments
Toluene was degassed by bubbling with N2 gas for 30 min.
The reaction mixture was analyzed by TLC (hexane : ethyl acetate = 1 : 1, Rf = 0.30) and GC.
Analytical Data (4-Methyl-N-phenylaniline)
1H NMR (400 MHz, CDCl3); δ 7.24-7.20 (t, J = 8.7 Hz, 3H), 7.07 (d, J = 8.7 Hz, 2H), 7.00-6.88 (m, 4H), 6.88-6.84 (m, 1H), 5.58 (s, 1H), 2.29 (s, 3H).
Lead Reference
- Tetramethoxybenzene is a Good Building Block for Molecular Wires: Insights from Photoinduced Electron Transfer
Used Chemicals
Procedure
To a 3-necked 300 mL round bottom flask was charged with diphenylamine (5.01 g, 29.6 mmol, 1.0 eq.), 4-chloroanisole (4.48 g, 31.4 mmol, 1.05 eq.) and degassed toluene (150 mL). To this solution was added Pd2(dba)3 (0.287 g, 0.131 mmol, 1 mol%), tri-tert-butylphosphonium tetrafluoroborate (0.198 g, 0.683 mmol, 2 mol%) and sodium tert-butoxide (6.34 g, 66.0 mmol, 2.2 eq.). The reaction mixture was refluxed for 16 hr under nitrogen atmosphere. After cooled to room temperature, the reaction was diluted with CH2Cl2 (300 mL). The suspension was filtered and the filtrate was dried over Na2SO4 and concentrated under reduced pressure to afford the crude and brown solid. The crude product was purified by silica-gel column chromatography (hexane/EtOAc = 99/1 then 8/1) to afford the light brown solid (7.0 g) containing 10 mol% of diphenylamine. Removal of the residual diphenylamine by recrystallization from hexane (55 mL, 60 °C then 15 °C) gave 4-methoxytriphenylamine as a white solid (5.26 g, 65 %).
Experimenter’s Comments
The reaction mixture was monitored by TLC (EtOAc/hexane = 1/10. Starting materials: Rf = 0.36 (diphenylamine), 0.59 (4-chloroanisole); target product: Rf = 0.46).
Analytical Data(4-Methoxytriphenylamine)
1H NMR (400 MHz, CD2Cl2); δ 7.26-7.17 (m, 4H), 7.10-6.98 (m, 6H), 6.98-6.91 (m, 2H), 6.89-6.82 (m, 2H), 3.79 (s, 3H).
13C NMR (101 MHz, CD2Cl2); δ 156.72, 148.56, 141.00, 129.39, 127.72, 123.12, 122.15, 115.05, 55.77.
Lead References
- Air-Stable Trialkylphosphonium Salts: Simple, Practical, and Versatile Replacements for Air-Sensitive Trialkylphosphines. Applications in Stoichiometric and Catalytic Processes
- An Air and Thermally Stable One- Component Catalyst for the Amination of Aryl Chlorides
Other References
- A Simple Catalytic Method for the Conversion of Aryl Bromides to Arylamines
- Palladium-catalyzed synthesis of arylamines from aryl halides. Mechanistic studies lead to coupling in the absence of tin reagents
- Air-stable trialkylphosphonium salts


References
[Product Highlights] Air-stable Tri-tert-butylphosphine Equivalent
[TCI Practical Example] Buchwald-Hartwig Amination Using Pd2(dba)3 and tBu3P·HBF4
SDS
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
示例 CoA
目前沒有該產品的 CoA 示例。
分析圖譜
很抱歉,您搜索的分析圖譜無法提供。





