Maximum quantity allowed is 999
请选择数量
CAS RN: 20353-93-9 | 產品號碼: G0393
產品規格
| Appearance | Light orange to Yellow to Green powder to crystal |
| Purity(HPLC) | min. 97.0 area% |
| Purity(Nonaqueous Titration) | min. 97.0 % |
| Melting point | 97.0 to 101.0 °C |
性質
| 熔點 | 99 °C |
| 溶解性(可溶於) | Methanol |
GHS
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防範說明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相關法規
運輸資料
| HS編碼* | 2925.29-000 |
Application
Synthesis of 4-Hydroxyquinazolines Using Gold’s Reagent
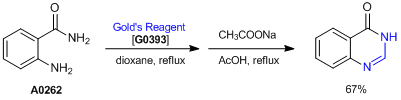
A dry, three-necked, round-bottomed flask is equipped with a condenser, thermometer, magnetic stirrer and is placed under a nitrogen atmosphere. Into the flask is placed 100 mL of dry dioxane and 6.8 g (0.05 mol) of 2-aminobenzamide and 9.9 g (0.061 mol) of Gold’s reagent. The resulting mixture is refluxed overnight and subsequently cooled to room temperature. Anhydrous sodium acetate (4.1 g, 0.05 mol) and glacial acetic acid (2 mL) are added and the resulting mixture is refluxed for 3 hr, cooled to room temperature, and the solvent is removed in vacuo. The solid residue is collected by vacuum filtration with the aid of a small amount (15 mL) of water. The solid is vacuum dried to yield 4.9 g (67% yield) of 4-hydroxyquinazoline.
References
- J. T. Gupton, K. F. Correia, B. S. Foster, Synth. Commun. 1986, 16, 365.

- J. T. Gupton, K. F. Correia, G. R. Hertel, Synth. Commun. 1984, 14, 1013.

Application
Formylation Reaction Using Gold’s Reagent

A round-bottomed flask is equipped with a magnetic stirrer, condenser and placed under a nitrogen atmosphere. The apparatus is flame dried and 8.3 g (0.05 mol) of Gold’s reagent is introduced along with 100 mL of dry THF. The mixture is cooled in an ice-water bath and 34 mL (0.054 mol) of a 1.6 M solution of n-octylmagnesium bromide in THF is added dropwise via syringe. The resulting mixture is refluxed for several hours and stirred overnight at room temperature. The reaction mixture is cooled in an ice-water bath and 70 mL of 10% aqueous hydrochloric acid is added. After stirring for 15 min, the mixture is neutralized with solid sodium bicarbonate and extracted with chloroform (3 x 80 mL). The combined chloroform extracts are dried over anhydrous sodium sulfate and concentrated to give 9.0 g of a brown oil. This material is distilled (Kugelrohr) to yield 5.6 g (79% yield) of a yellow oil.
References
Application
Synthesis of 5-Membered Heterocyclic Rings Using Gold’s Reagent

References
- 1)One Atom Lynch Pin Transformations of Gold's Reagent
- 2)New Synthesis of 1,5-Disubstituted Imidazoles
考研文獻
產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜
請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。





