Maximum quantity allowed is 999
CAS RN: 12148-71-9 | 產品號碼: C2662
(1,5-Cyclooctadiene)(methoxy)iridium(I) Dimer

* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | C2662 |
| 分子式 / 分子量 | C__1__8H__3__0Ir__2O__2 = 662.87 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Frozen (<0°C) |
| 儲存在惰性氣體下 | Store under inert gas |
| 應避免的情況 | Light Sensitive,Air Sensitive,Moisture Sensitive,Heat Sensitive |
| 包裝和容器 | 1G-Glass Bottle with Plastic Insert (閲覽圖片), 200MG-Glass Bottle with Plastic Insert (閲覽圖片) |
| CAS RN | 12148-71-9 |
| Reaxys-RN | 14520157 |
| PubChem Substance ID | 160871404 |
| MDL編號 | MFCD08459360 |
| Appearance | Light yellow to Amber to Dark green powder to crystal |
| Elemental analysis(Carbon) | 31.50 to 34.00 % |
| 熔點 | 179 °C(dec.) |
| HS編碼* | 2843.90-000 |
-
Used Chemicals
-
Procedure
-
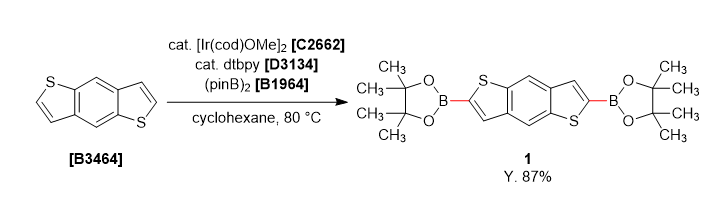
A solution of dtbpy (27 mg, 0.05 mmol), bis(pinacolato)diboron (1.0 g, 4.0 mmol) and [Ir(cod)OMe]2 (33 mg, 0.025 mmol) in cyclohexane (40 mL) was stirred under nitrogen at room temperature for 10 min. Benzo[1,2-b:4,5-b']dithiophene (380 mg, 2.0 mmol) was added the mixture and stirred 80 ˚C for 18 hours. The reaction mixture was quenched with water and separated both layers, extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the crude was washed with methanol (20 mL) to give 1 as a white solid (0.771 g, 87% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by NMR.
Cyclohexane was bubbled with nitrogen before use.
-
Analytical Data
-
Compound 1
1H NMR (270 MHz, CDCl3); δ 8.36 (s, 2H), 7.90 (s, 2H), 1.39 (s, 24H).
-
Lead Reference
-
- Synthesis and Transistor Application of Bis[1]benzothieno[6,7‑d:6′,7′‑d′]benzo[1,2‑b:4,5‑b′]dithiophenes

Reference
- Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent

An alcohol or ketone substrate is dissolved in THF and treated with a freshly prepared solution of [Ir(cod)OMe]2 (0.05 mol%) in THF and then with neat Et2SiH2 (1.2 eq.). The resulting solution is stirred at room temperature (23 °C) until complete conversion of the alcohol or ketone. At the completion of the reaction, the corresponding diethyl(hydrido)silyl ether is observed. Then the reaction mixture is placed under high vacuum for 1 h. The concentrated diethyl(hydrido)silyl ether is sequentially treated with freshly prepared solutions of norbornene (1.2 eq.) in THF and [Ir(cod)OMe]2 (0.5 mol%) in THF, and then with a slurry of Me4phen (1.25 mol%) in THF. The resulting solution is stirred at room temperature for 1 h and then heated it at 80-120 °C until complete conversion to the corresponding oxasilolane is observed. Then the crude reaction mixture containing the oxasilolane is sequentially treated with MeOH, KHCO3 (2.5 eq.) and H2O2 (30% solution in H2O, 10 eq.), and the resulting mixture is stirred overnight at 50 °C. The reaction is carefully quenched with aq. NaHSO3, and the resulting mixture is extracted with EtOAc. The combined organic layer is sequentially washed with 1 M HCl and sat. NaHCO3, and then dried with MgSO4. The resulting organic layer is filtered through Celite and concentrated to provide the crude diol, which is either purified directly or after conversion to the corresponding acetate derivative through treatment with Ac2O and Et3N.
References
[Research Articles] Catalytic Functionalization of Un-activated Primary C-H Bonds
SDS
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
示例 CoA
目前沒有該產品的 CoA 示例。
分析圖譜
很抱歉,您搜索的分析圖譜無法提供。




