Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [Product Highlights] Cell Culture-Ready Antibiotic... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
CAS RN: | 產品號碼: C2637
Copper(II) on Chitosan (Cu 1.3mmol/g)
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | C2637 |
| 外觀與形狀(20°C) | Solid |
儲存條件 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
產品規格
| Appearance | Green to Dark green to Dark blue powder to crystal |
| Loading | 1.2 to 1.5 mmol/g |
| Functionality test | to pass test |
性質
GHS
相關法規
運輸資料
| HS編碼* | 3822.19-000 |
Application
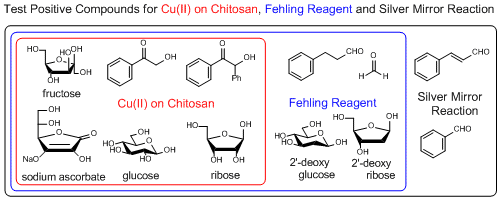
Copper (II) on Chitosan, a reagent for selective discrimination between 2-hydroxy and 2-deoxy sugars, an alternative test reagent for Fehling and Benedict reagents
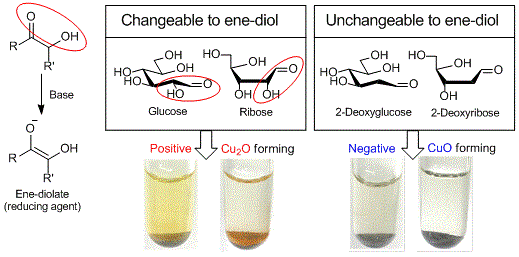
Copper (II) on chitosan is a chitosan-supported copper (II) (about 1.3 mmol/g) developed by Inoue et al. as a reagent for selective detection of reducible organic compounds. The Cu (II) on chitosan can selectively detect reducible organic compounds bearing structures changeable to ene-diolate (e.g. glucose, ribose) in basic aqueous solution with the formation of copper (I) oxide (Cu2O) as a signal of the reduction by the ene-diolate.

(The color of Cu (II) on chitosan is subject to change to green after opening. But the green-colored Cu (II) on chitosan changes to dark blue which is suitable for this detection in basic aqueous solution, in the same way as a new reagent.)
Typical procedure (detection of a sugar): A test tube (15 mm inside diameters) is charged with copper (II) on chitosan (20 mg) and an aqueous solution of 0.5 mol/L NaOH (1 mL). At this point, the color of copper (II) on chitosan changes from blue to dark blue. Then, an aqueous solution of 10 mmol/L sugar (4 mL) is added, and the mixture is stirred at 70 to 80 °C for 5 min.
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).

Using Cu (II) on chitosan, needs a lesser amount of Cu (II) than that of Fehling and Benedict reagents and leads to significantly reducing the reagent costs and waste chemicals. In addition, Cu (II) on chitosan is a powdery substance, which is easily handled and free from leaking in carrying and storage. Notably, Cu (II) on chitosan is suitable for science educational experiments such as a test for the detection of a reducing sugar yielded from starch hydrolysis.

References
- S. Ogura, M. Inoue, Kagaku to Kyoiku (Chemical Education) 2013, 61, 86. (Japanese)
產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜

請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。






