Maximum quantity allowed is 999
CAS RN: 53199-31-8 | 產品號碼: B3161
Bis(tri-tert-butylphosphine)palladium(0)
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | B3161 |
| 純度/分析方法 | >98.0%(T) |
| 分子式 / 分子量 | C__2__4H__5__4P__2Pd = 511.06 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Frozen (<0°C) |
| 儲存在惰性氣體下 | Store under inert gas |
| 應避免的情況 | Air Sensitive,Heat Sensitive |
| 包裝和容器 | 1G-Glass Bottle with Plastic Insert (閲覽圖片), 250MG-Glass Bottle with Plastic Insert (閲覽圖片) |
| CAS RN | 53199-31-8 |
| Reaxys-RN | 14300595 |
| PubChem Substance ID | 87560386 |
| MDL編號 | MFCD03094580 |
| Appearance | White to Yellow to Orange powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
| HS編碼* | 2843.90-000 |
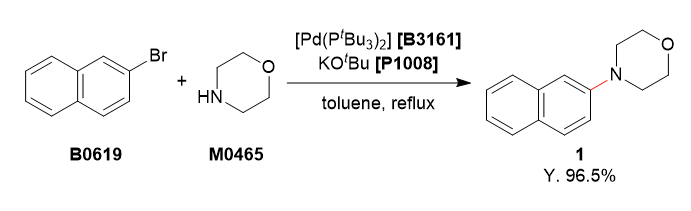
Used Chemicals
Procedure
To a 4-necked 200 mL flask was charged with 2-bromonaphthalene (5.20 g, 25.1 mmol, 1.0 equiv.), morpholine (3.27 mL, 37.5 mmol, 1.5 equiv.) and degassed toluene (75 mL). To this solution was added bis(tri-tert-butylphosphine)palladium(0) (256 mg, 0.501 mmol, 2.0 mol%) and potassium tert-butoxide (4.21 g, 37.5 mmol, 1.5 equiv.). The reaction mixture was refluxed for 3 h under argon atmosphere. The reaction mixture was cooled to room temperature and washed with water (50 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (50 mL). The combined organic layers were washed with brine (50 mL), dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue was purified by column chromatography (eluent: hexane/ethyl acetate, 85/15→70/30) to obtain 1 as a white solid (5.17 g, 96.5%).
Experimenter's Comments
The reaction mixture was monitored by TLC (hexane/ethyl acetate = 9/1, Rf = 0.70).
Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 7.76–7.69 (m, 3H), 7.42 (t, J = 7.2 Hz, 1H), 7.31 (t, J = 6.8 Hz, 1H), 7.25–7.28 (m, 1H), 7.13 (s, 1H), 3.92 (t, J = 4.8 Hz, 2H), 3.27 (t, J = 4.8 Hz, 2H).
13C NMR (101 MHz, CDCl3); δ 129.0, 127.6, 127.0, 126.5, 123.7, 119.1, 110.3, 67.1, 50.0.
Lead Reference
- Heterogeneous Rhodium‐Catalyzed Aerobic Oxidative Dehydrogenative Cross‐Coupling: Nonsymmetrical Biaryl Amines
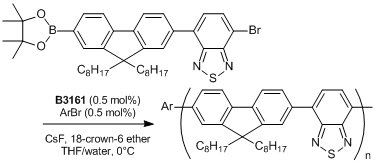
References
- Chain-Growth Suzuki Polymerization of n-Type Fluorene Copolymers
- E. Elmalem, A. Kiriy, W. T. S. Huck, Macromolecules 2011, 44, 9057.


Typical Procedure: An ampoule is charged with the thiocarbamate (0.442 mmol) and anhydrous degassed toluene (4 mL) is added via gastight syringe under nitrogen with stirring. A J. Youngs valve is fitted and the ampoule is placed into an oil bath pre-equilibrated at 100℃. After ca. 5 minutes the valve is removed and Pd(t-Bu3P)2 (2 mol%, 0.00884 mmol) is added as a solid, the valve is then replaced and the reaction mixture heated for 2.5 hours. A sample of the reaction mixture is removed via gastight syringe and found to contain the desired product (>99%).
Reference
- The Newman–Kwart Rearrangement of O‐Aryl Thiocarbamates: Substantial Reduction in Reaction Temperatures through Palladium Catalysis
文章/手冊
SDS
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
示例 CoA
目前沒有該產品的 CoA 示例。
分析圖譜
很抱歉,您搜索的分析圖譜無法提供。





