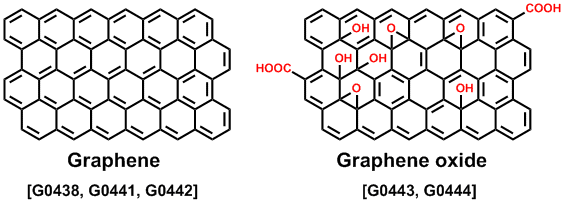
The fabrication procedure for graphene is peeling off the layer from HOPG, a chemical vapor deposition (CVD)7) as well as reduction of a graphene oxide (GO).8) There are various synthetic methods of making GO, and the properties and applications depend on the degree of oxidation. GO disperses in water and several polar solvents, because the structure of GO normally includes hydroxyl, epoxy, and carboxyl groups on the graphene sheet. Accordingly, a GO thin film can be fabricated on a substrate by a solution-process. The reduction of GO provides a reduced graphene oxide (rGO), but it is not a perfect graphene. The rGO contains a few oxygen components and defects on the graphene structure. Although GO is an insulator because there are sp3 carbon atoms, rGO is conductive. Therefore the rGO is expected to be an electrode material. A water dispersion of GO is used as a lubricant to reduce friction on metal surfaces.9) GO-supported metal catalysts were developed for a cross-coupling reaction and hydrogenation.10,11) We can introduce several functional groups on GO because there are oxygen-based groups. These GO derivatives may be useful for luminescent materials and biosensors.12,13)
Maximum quantity allowed is 999
请选择数量
| 產品號碼 | G0500 |
| CAS RN | 1034343-98-0 |
| 純度/分析方法 |
| 產品號碼 | G0501 |
| CAS RN | 1034343-98-0 |
| 純度/分析方法 |
| 產品號碼 | G0502 |
| CAS RN | 1034343-98-0 |
| 純度/分析方法 |
| 產品號碼 | G0670 |
| CAS RN | 1034343-98-0 |
| 純度/分析方法 |
| 產品號碼 | G0671 |
| CAS RN | 1034343-98-0 |
| 純度/分析方法 |
產品號碼: G0500 |
純度/分析方法
產品號碼: G0501 |
純度/分析方法
產品號碼: G0502 |
純度/分析方法
產品號碼: G0670 |
純度/分析方法
添加到您的購物車
Product Availability by Store Location
Hours