Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
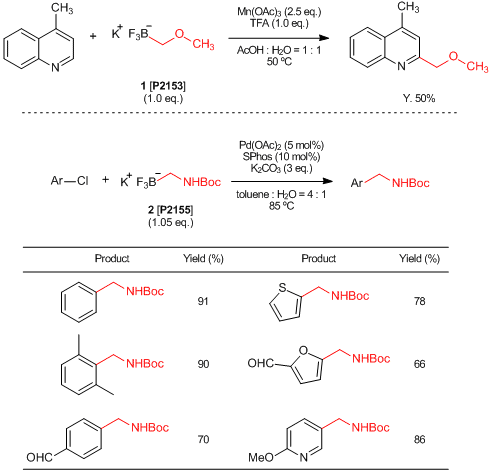
Efficient Trifluoroborates Usable for Introducing Methoxymethyl and Aminomethyl Groups
Organoboronic acids are commonly used reactants for Suzuki-Miyaura cross-coupling reactions. Generally, aryl boronic acids and alkenyl boronic acids are chemically stable and easy to treat while alkyl boronic acids are less stable and some of them are difficult to synthesize. Trifluoroborates are one of the organic boron compounds which are more electrically and chemically stable due to full substitution on their boron atom with fluorine atoms. For instance, potassium (methoxymethyl)trifluoroborate (1) and potassium (N-Boc-aminomethyl)trifluoroborate (2) are stable and solid trifluoroborate derivatives which can be used for introducing methoxymethyl1) and aminomethyl2) groups by transition-metal catalyzed cross-coupling reactions.
References
- 1)Direct Alkylation of Heteroaryls Using Potassium Alkyl- and Alkoxymethyltrifluoroborates
- 2)Synthesis and Suzuki–Miyaura Cross-Coupling Reactions of Potassium Boc-Protected Aminomethyltrifluoroborate with Aryl and Hetaryl Halides