Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [TCIPracticalExample] Suzuki-Miyaura Coupling Using Encapsulated... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 247089-85-6 | Product Number: T2814
(R)-(-)-p-Toluenesulfinamide
Purity: >98.0%(HPLC)
Synonyms:
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 200MG |
NT$2,856
|
3 | 0 | Contact Us | |
| 1G |
NT$9,660
|
7 | 1 | Contact Us |
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | T2814 |
Purity / Analysis Method 
|
>98.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__7H__9NOS = 155.22 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 247089-85-6 |
| Reaxys Registry Number | 8110323 |
| PubChem Substance ID | 354334152 |
| MDL Number | MFCD06858374 |
Specifications
| Appearance | White to Orange to Green powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(with Total Nitrogen) | min. 97.0 % |
| Melting point | 116.0 to 120.0 °C |
| Specific rotation [a]20/D | -81.0 to -91.0 deg(C=1, CHCL3) |
Properties (reference)
| Melting Point | 118 °C |
| Specific Rotation | -86° (C=1,CHCl3) |
| Solubility (soluble in) | Methanol |
GHS
Related Laws:
Transport Information:
| H.S.code* | 2930.90-900 |
Application
Sufinamide for the Synthesis of Chiral Amines
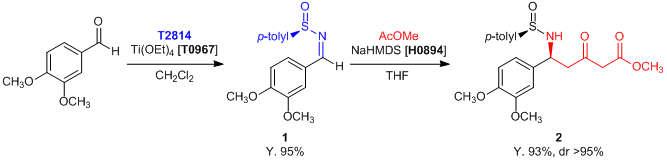
Experimental procedure (1 to 2): In a flask equipped with argon is placed a solution of THF (100 mL) and NaHMDS (1.0 mol/L solution in THF, 148 mL, 148 mmol, 6 eq.) at -78 °C. Methyl acetate (7.85 mL, 98.8 mmol, 4 eq.) in THF (20 mL) is added slowly, and the solution is stirred for 1 h. At this time anhydrous ether (20 mL) and 1 (7.48 g, 24.7 mmol, 1 eq.) in THF (20 mL) are added. The reaction mixture is stirred at -78 °C for 3.5 h and quenched with sat. NH4Cl (50 mL). The solution is washed with ethyl acetate (3 x 150 mL), the organic phases are combined, dried (Na2SO4), and concentrated. Purification by flash chromatography (50% EtOAc/hexane) gives 2 (8.80 g, 85% yield) as an oil.
References
- Alkaloid Synthesis Using Chiral δ-Amino β-Ketoesters: A Stereoselective Synthesis of (-)-Lasubine II
PubMed Literature
Documents
Product Articles
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




