Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [Product Highlights] Cell Culture-Ready Antibiotic... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 17185-29-4 | Product Number: C1383
Carbonylhydridotris(triphenylphosphine)rhodium(I)
Purity:
Synonyms:
- Carbonyltris(triphenylphosphine)rhodium(I) Hydride
- Tris(triphenylphosphine)rhodium(I) Carbonyl Hydride
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 1G |
NT$8,400
|
1 | 0 | Contact Us | |
| 5G |
NT$32,466
|
12 | 4 | Contact Us |
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | C1383 |
| Molecular Formula / Molecular Weight | C__5__5H__4__6OP__3Rh = 918.80 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Moisture Sensitive |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 17185-29-4 |
| PubChem Substance ID | 87566394 |
| SDBS (AIST Spectral DB) | 39303 |
| Merck Index (14) | 9758 |
| MDL Number | MFCD00151644 |
Specifications
| Appearance | Light yellow to Yellow powder to crystal |
| Elemental analysis(Carbon) | 70.50 to 72.60 % |
Properties (reference)
| Melting Point | 122 °C(dec.) |
| Solubility (soluble in) | Chloroform, dichloromethane, Benzene |
GHS
| Hazard Statements | H413 : May cause long lasting harmful effects to aquatic life. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P273 : Avoid release to the environment. |
Related Laws:
Transport Information:
| H.S.code* | 2843.90-000 |
Application
Rh(I)-catalyzed synthesis of indoles through the amino-Claisen rearrangement of N-propargylanilines
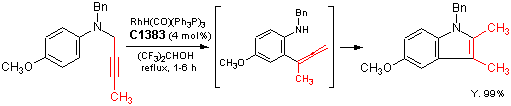
Typical procedure: Under an argon atmosphere, to a solution of carbonyltris(triphenylphosphine)rhodium(I) hydride (16 micro-mol) in 1,1,1,3,3,3-hexafluoro-2-propanol (1 mL) is added N-2-butynyl-N-benzyl-4-methoxyaniline (0.4 mmol) in 1,1,1,3,3,3-hexafluoro-2-propanol (1.5 mL) at an ambient temperature, and the mixture is refluxed until the consumption of the substrate by TLC analysis. The mixture is diluted with ether and filtered through a Celite pad. After concentration of the filtrate to dryness, purification by silica gel chromatography (hexane/AcOEt = 25:1) gives the corresponding indole in 99% yield.
References
PubMed Literature
Documents
Product Articles
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




