Maximum quantity allowed is 999
CAS RN: 14338-32-0 | Product Number: C0903
2-Chloro-1-methylpyridinium Iodide

Purity: >98.0%(T)
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | C0903 |
| Purity / Analysis Method | >98.0%(T) |
| Molecular Formula / Molecular Weight | C__6H__7ClIN = 255.48 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Light Sensitive,Hygroscopic |
| CAS RN | 14338-32-0 |
| Reaxys Registry Number | 3572320 |
| PubChem Substance ID | 87565959 |
| SDBS (AIST Spectral DB) | 10163 |
| Merck Index (14) | 6301 |
| MDL Number | MFCD00011984 |
| Appearance | Light yellow to Amber to Dark green powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Nonaqueous Titration) | min. 98.0 % |
| Solubility in Water | almost transparency |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| H.S.code* | 2933.39-000 |

-
Used Chemicals
-
Procedure
-
Boc-Pro-OH (1.65 g, 7.65 mmol), 2-chloro-1-methylpyridinium iodide (1.95 g, 7.65 mmol) were added to solution of H-Phe-OMe・HCl (1.50 g,6.95 mmol) in dichloromethane (30 mL) at room temperature and triethylamine (3.40 mL, 24.3 mmol) was added dropwise in 3 min at 5 to 10 °C. After stirring for 3 h, solvent was removed under reduced pressure and the residue was dissolved in ethyl acetate and washed with 10% citric acid aq. (30 mL × 3), 5% sodium bicarbonate solution aq. (30 mL × 3) and brine (30 mL). The organic layer was dried over anhydrous sodium sulfate and filtered. After the removal of solvent under reduced pressure, the given crude was purified by column chromatography (hexane : EtOAc = 2 : 1) to give Boc-Pro-Phe-OMe (2.37 g,91%) as a slightly yellowish solid.
-
Experimenter's Comments
-
Consumption of stating material and formation of product was monitored by LCMS.
-
Analytical Data(Boc-Pro-Phe-OMe)
-
1H NMR (400 MHz, DMSO-d6); δ 8.17–8.27 (m, 1H), 7.19–7.29 (m, 5H), 4.42–4.53 (m, 1H), 4.00–4.13 (m, 1H), 3.56–3.61 (m, 3H), 3.18–3.38 (m, 2H), 2.90–3.08 (m, 2H), 1.91–2.11 (m, 1H), 1.56–1.71 (m, 3H), 1.15-1.40 (m, 9H).
-
Lead Reference
-
- A facile synthesis of carboxamides by using 1-methyl-2-halopyridinium iodides as coupling reagents
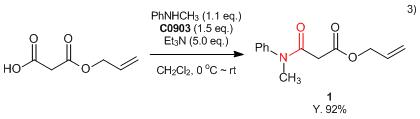
References
- 1) A CONVENIENT METHOD FOR THE SYNTHESIS OF CARBOXYLIC ESTERS
- 2) A FACILE SYNTHESIS OF CARBOXAMIDES BY USING 1-METHYL-2-HALOPYRIDINIUM IODIDES AS COUPLING REAGENTS
- 3) Asymmetric Decarboxylative Allylation of Oxindoles
Articles/Brochures
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.




