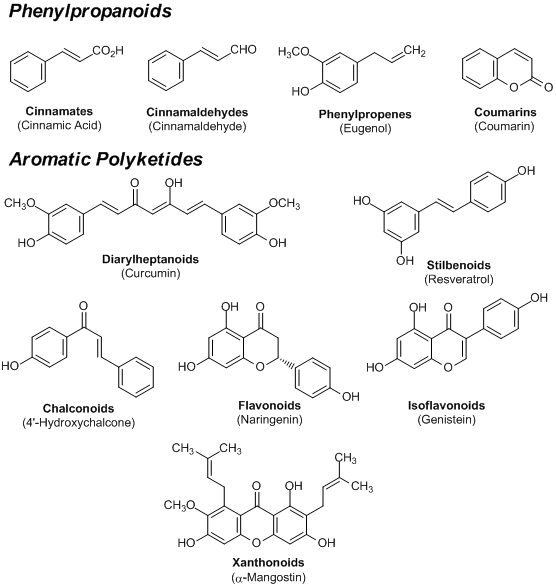
• Solubility
They are generally soluble in many organic solvents. They can be rather di? cult to dissolve in non-polar solvents such as hexane but dissolve well in high polar solvents such as chloroform, methanol and DMSO. Compounds with carboxyl or phenolic hydroxy groups are soluble in aqueous alkaline solutions. Since they are easily oxidized in the liquid state, we suggest you to use them within a short period of time after preparation.
Maintenance Notice (11:00 PM February 14 - 1:30 AM February 15, 2026 UTC): The website is scheduled for maintenance. The website will be available, but some functions may experience errors. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News Feburary 2026 | [Product Highlights] rac-GR24: A Standard Tool for ... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)