Haruhiko Taguchi
Tokyo Chemical Industry, Co. Ltd.
Maximum quantity allowed is 999
Please select the quantity
Short Topic: More ways to use reagents |
Mitsunobu Reaction Using Acetone Cyanohydrin
No.156(January 2013)
Organic Synthesis Using Acetone Cyanohydrin
This chat for introduction of another usage of reagents has started since issue #155 of TCIMAIL. In this issue, we pick acetone cyanohydrin. Acetone cyanohydrin has been used since the early 20th century, so various usages of it have been developed.1) As typical usages, cyanohydrynations of aldehydes and ketones, chemical synthesis of α-amino acids by the Strecker reaction and 1,4-additions of α,β-unsaturated carbonyl compounds have been performed by using acetone cyanohydrin. Acetone cyanohydrin can be also used as a source of the cyano anion and which reacts with alkyl halides to afford corresponding products. Furthermore, in industrial usage, it has been used for the intermediate in the production of poly(methyl methacrylate) resin.
_web.jpg)
As described above, acetone cyanohydrin has a number of usages. However, it is considered that acetone cyanohydrin is a minor item compared with other cyanation reagents such as sodium cyanide and potassium cyanide because most chemists will chose them at the beginning of trying a cyanation. At first, I will describe the synthetic properties of alkanenitriles by using such cyanation reagents.
Efficient Synthetic Methods of Secondary Alkanenitriles
The synthesis of alkanenitriles is commonly seen in various textbooks of organic chemistry. This process seemed to be easy for us. I had thought the synthesis of alkanenitriles was very easy only being careful of treatment of the cyanide ion in graduate studies. But actually I had tried to synthesize alkanenitriles. The synthesis of them was very tough and much liquid waste containing cyanide ion had been produced.
In general, alkanenitriles will be synthesized by the reaction of alkyl halides with cyanide ion. It is true that such a reaction is widely suited when primary alkyl halides are employed, but when secondary alkyl halides are employed, the yields decreased in some cases depending on these structures. It is considered that the nucleophilic and basic characters of a cyano ion are present at the same time when using secondary alkyl halides, so nucleophilic substitution of a cyano ion wouldn’t proceed preferentially. Of course, when tertiary alkyl halides are used, the cyanation isn’t successful. These alkylations using cyano ion are good examples for study of the nucleophilic and basic characters of the cyano ion from actual chemical experiments.
Well, how can secondary alkanenitriles be synthesized effectively? One successful method is shown in technical books of organic synthesis, in which generation of the α-anion of primary alkanenitriles by the action of sodium amide in liquid ammonia followed by using it for the synthesis of secondary alkanenitriles. This synthetic method is excellent but many special tools are needed to prepare liquid ammonia and more, it is hard work. If there are other synthetic methods, most chemists will select another one.
Another method focuses on the α-proton of alkanenitriles with a pKa value commonly of 25. This result suggests that an α-proton of alkanenitriles can be removed by the action of a strong base such as LDA. Consequent treatment with alkyl halides will form secondary alkanenitriles. So when I actually tried according to such a synthetic manner, it gave only a tertiary alkanenitrile. There was no observation of any secondary alkanenitriles. I considered that in this synthetic method, the pKa value of monoalkylated alkanenitriles increases compared to non-alkylated alkanenitriles, so the second deprotonation of mono-alkylated alkanenitriles occurs more rapidly forming products with a second alkylation; that is, a tertiary alkanenitrile is formed as a sole product. I think that this synthetic method is excellent because it can be used for the synthesis of tertiary alkanenitriles.
Thus, for the conventional method for the synthesis of secondary alkanenitriles, I think alkylation of cyanoacetate esters is better. After alkylation, hydrolysis of the ester group and consequent decarboxylation, the desired secondary alkanenitriles will be given. If the hydrolysis of alkylated cyanoacetate esters is performed under alkaline conditions, the cyano group will be partially hydrolyzed. Of course, after work up, the desired alkanenitriles are obtained but the yields would be a little decreased. To optimize the above synthetic manner, after alkylation, decarboxylation is performed by Krapcho’s decarboxylation2) instead of an alkaline hydrolysis consequent decarboxylation. I believe this method would be one of the most effective synthetic methods of secondary alkanenitriles.
Alkanenitriles are well used for organic synthesis because the cyano group can be easily exchanged to other functional groups. But their chemical synthesis is not simple and the development of an efficient synthetic route to them is very hard.
To Use Acetone cyanhydrin for the Mitsunobu Reaction
Well, let's get back to the subject, as a remarkable character of acetone cyanohydrin, which shows formally the same reactivities as hydrogen cyanide. Here, focusing on the pKa value of hydrogen cyanide, it is about 9.1. It is suggested that hydrogen cyanide can be used as a reactant in the Mitsunobu reaction. Hydrogen cyanide is in the liquid or gas state at ambient temperature making it difficult to use it for the Mitsunobu reaction. For such a usage, acetone cyanohydrin should be used instead of hydrogen cyanide. Tsunoda and his coworkers searched a cyanation of alcohols by the Mitsunobu reaction using an acetone cyanohydrin and found primary and secondary alcohols are successfully transformed to the corresponding alkanenitriles in good to moderate yield with an inversion of stereochemistry.3)
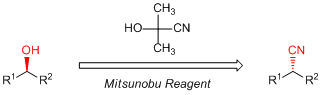
Further searching of references about the Mitsunobu reaction using acetone cyanohydrin, which is well used by pharmaceutical companies in syntheses of drug substances, is warranted. It seems that the Mitsunobu reaction is a useful synthetic method for such a purpose because it has wide range of applications and the reactions proceed under mild conditions. These synthetic advantages would be fit for the synthesis of drug substances.
TCI has various reagents which can be used for the Mitsunobu reaction. Especially, the Tsunoda reagent is widely suited for various Brønsted acids because the low pKa value can be employed. So, the Mitsunobu reaction using acetone cyanohydrin is a very attractive synthetic method for introducing a cyano group. All of reagents are available from TCI.
References
- 1) S. A. Haroutounian, in Encyclopedia of Reagents for Organic Synthesis, ‘Acetone Cyanohydrin’ 2001, 28.
- 2) a) A. P. Krapcho, Synthesis 1982, 805. b) A. P. Krapcho, Synthesis 1982, 893.

- 3) T. Tsunoda, K. Uemoto, C. Nagino, M. Kawamura, H. Kaku, S. Ito, Tetrahedron Lett. 1999, 40, 7355.

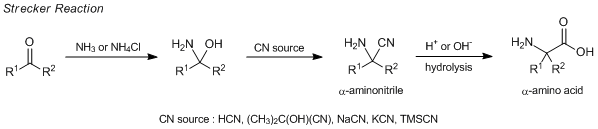
The Strecker reaction is one of the methods for the synthesis of α-amino acids and which has been conventionally used since the middle of the 19th century. The Strecker reaction is performed by the following: Aldehydes or ketones are reacted with hydrogen cyanide in the presence of ammonia or ammonium chloride to afford the corresponding α-aminonitriles, which are directly transformed to α-amino acids by alkaline- or acid-hydrolysis. Acetone cyanohydrin, potassium cyanide, sodium cyanide and trimethylsilyl cyanide are used as a cyanide source instead of hydrogen cyanide.
The Krapcho reaction is the method for the decarboxylation of carboxylic acid esters having an electron-withdrawing group at the β-position without hydrolysis of the ester group. In this synthetic manner, DMSO or DMSO-water is used as a solvent and the decarboxylation proceeds more rapidly by addition of salts such as sodium chloride, lithium chloride, potassium cyanide, or sodium cyanide. The detail of this reaction and a number of experimental data are collected in “Synthesis” by Krapcho.1)

Related Compounds
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.