Maximum quantity allowed is 999
Please select the quantity
Direct Cyanomethylation of Nitroarenes
No.126(August 2007)
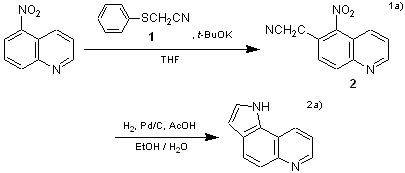
(Phenylthio)acetonitrile (1) can react with nitroarenes and introduce a cyanomethyl group at the ortho- or para- position to the nitro group.1 The resulting o-nitroarylacetonitrile 2, for example, is a useful starting material for the synthesis of nitrogen-containing fused ring compounds.2
References
- 1)Vicarious substitution of hydrogen in aromatic nitro compounds
- 2)The synthesis of N-containing fused ring compounds
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.