Maintenance Notice (10:30 PM June 7 - 2:00 AM June 8, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News June 2025 | [Product Highlights] Benzoquinone Derivative Useful for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
Ingenol: Highly Strained Natural Product as a Core of Powerful Bioactive Compounds
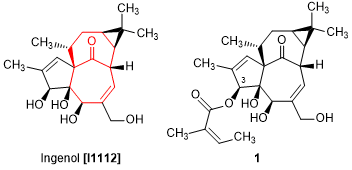
Ingenol is a diterpene isolated from Euphobia family and its structure was determined in 1970.1) Although ingenol has a weak activity against protein kinase C,2) ingenol derivatives show wide range of bioactivity. For instance, ingenol 3-mebutate, an angelate ester at 3-hydroxy group of ingenol, is utilized as a therapeutic medicine for solar keratosis (a precancerous skin condition).3) The mechanism of 1 is considered as an activation of protein kinase C, so it has also been reported to indicate an anticancer4) and anti-HIV5) activities. By the way, ingenol has an “inside-outside structure”, a unique and highly strained structure at a condensed ring moiety of two seven-membered rings, so that it is regarded as a challenging target of total synthesis.6,7)
Related Products
References
- 1)Structure determination of the new tetracyclic diterpene ingenol-triacetate with triple product methods
- 2)Specific Binding to Protein Kinase C by Ingenol and Its Induction of Biological Responses
- 3)Ingenol Mebutate Gel 0.015% and 0.05%: In Actinic Keratosis
- 4)The Protein Kinase C Agonist PEP005 (Ingenol 3-Angelate) in the Treatment of Human Cancer: A Balance between Efficacy and Toxicity
- 5)HIV Type 1 Inhibition by Protein Kinase C Modulatory Compounds
- 6)Total Synthesis of Ingenol
- 7)14-Step Synthesis of (+)-Ingenol from (+)-3-Carene