Maintenance Notice (3:00 AM - 8:30 AM November 1, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News October 2025 | [Product Highlights] Tetraphenylethylene Derivatives Used for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
CAS RN: 1109-15-5 | 产品编码: T2313
Tris(pentafluorophenyl)borane

| 产品编码 | T2313 |
纯度/分析方法 
|
>98.0%(NMR) |
| 分子式/分子量 | C__1__8BF__1__5 = 511.98 |
| 外观与形状(20°C) | 固体 |
储存温度 
|
冷冻 (<0°C) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 湿气 (吸湿),加热 |
包装和容器 
|
1G-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 1109-15-5 |
| Reaxys-RN | 2931347 |
| PubChem物质ID | 87558752 |
| Merck Index (14) | 9755 |
| MDL编号 | MFCD00269813 |
技术规格
| Appearance | White to Gray to Brown powder to crystal |
| Purity(NMR) | min. 98.0 atom% |
| Water | max. 1.0 % |
物性(参考值)
| 熔点 | 128 °C |
| 最大吸收波长 | 306 nm (Toluene) |
| 溶解性(可溶于) | 甲苯 |
GHS
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相关法规
运输信息
| HS编码* | 2931.90-000 |
应用
Deuteration of Aromatic and Heteroamomatic Compounds Catalyzed by Tris(pentafluorophenyl)borane
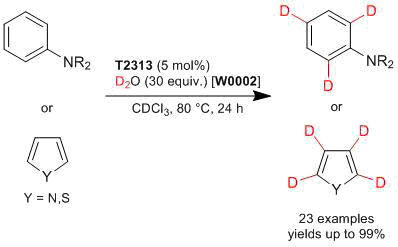
Experimental procedure: N,N-Dibenzylaniline (140 mg, 0.513 mmol), tris(pentafluorophenyl)borane (17.0 mg, 0.033 mmol), D2O (306 mg, 15.3 mmol) are placed in a sealed tube. The reaction mixture is stirred at 80 °C for 24 h. After the reaction is completed, the reaction mixture is purified by silica gel column chromatography (cyclohexane:ethylacetate = 20:1) to afford N,N-dibenzylaniline-2,4,6-d3 (134 mg) in 95% yield.
References
- B(C6F5)3‑Catalyzed Regioselective Deuteration of Electron-Rich Aromatic and Heteroaromatic Compounds
应用
High Throughput Sequence-controlled Oligosiloxane Synthesis

References
- By-Product-Free Siloxane-Bond Formation and Programmed One-Pot Oligosiloxane Synthesis
应用
Frustrated Lewis Pair (FLP)-induced Hydrogenations of Silyl Enol Ethers

References
- Heterolytic dihydrogen activation with the 1,8-bis(diphenylphosphino)naphthalene/B(C6F5)3 pair and its application for metal-free catalytic hydrogenation of silyl enol ether
应用
Tri(cyclohexa-2,5-dien-1-yl)silane: A Stable and Easy-to-handle Surrogate of Monosilane (SiH4)

Synthesis of multisubstituted silanes:
In a glove box, a Schlenk tube is charged with an alkene (0.300 mmol). In two vials are weighed B(C6F5)3 (7.7 mg, 15 µmol) and tri(cyclohexa-2,5-dien-1-yl)silane (1, 28.0 mg, 0.105 mmol). All the sealed containers are taken out of the glove box, and the Schlenk tube is connected to a N2 line. Under a N2 counterflow are subsequently added 0.3 mL of CH2Cl2, 1 then B(C6F5)3. The tube is completely sealed and stirred at room temperature for 24 h. After this time, two drops of triethylamine are added to the solution under an N2 counterflow, and the resulting mixture is stirred for 5 min before being filtered over a short alumina column (elution with CH2Cl2). After evaporation of all volatiles, the crude product is purified by flash column chromatography to give the multisubstituted silane 3.
In a glove box, a Schlenk tube is charged with an alkene (0.300 mmol). In two vials are weighed B(C6F5)3 (7.7 mg, 15 µmol) and tri(cyclohexa-2,5-dien-1-yl)silane (1, 28.0 mg, 0.105 mmol). All the sealed containers are taken out of the glove box, and the Schlenk tube is connected to a N2 line. Under a N2 counterflow are subsequently added 0.3 mL of CH2Cl2, 1 then B(C6F5)3. The tube is completely sealed and stirred at room temperature for 24 h. After this time, two drops of triethylamine are added to the solution under an N2 counterflow, and the resulting mixture is stirred for 5 min before being filtered over a short alumina column (elution with CH2Cl2). After evaporation of all volatiles, the crude product is purified by flash column chromatography to give the multisubstituted silane 3.
References
- Formal SiH4 chemistry using stable and easy-to-handle surrogates
应用
Metal-Free Hydrogenation of Ketone by Frustrated Lewis Pairs (FLPs)

Typical procedure (R, R’ = n-Pr): 4-Heptanone (1.00 g, 8.76 mmol) is weighed into a 125 mL reactor. Subsequently, B(C6F5)3 (0.224 g, 0.43 mmol) dissolved in Et2O (14.3 mg, 20 mL, 0.19 mol) is added to the reactor. The reactor is sealed and attached to a hydrogen gas line. The flask is purged ten times at 15 atm with hydrogen gas. The reactor is then pressurized with 60 atm hydrogen gas and placed in an oil bath for 12 h at 70 °C. The reactor is slowly vented and all the volatiles are collected by vacuum distillation while cooling the collected distillate with liquid nitrogen. The solvent is removed by applying a gentle stream of N2 gas to give 4-heptanol (886 mg, 87% yield, and 99 % conversion determined by 1H NMR).
References
- T. Mahdi, D. W. Stephan, J. Am. Chem. Soc. 2014, 136, 15809.

- Highlighted in Chem. Eng. News 2014, 92 (44), 8.

应用
Metal-Free Hydrogenation of N-Phenyl Aromatic Rings

Typical procedure (Entry 1): A 25 mL glass bomb equipped with Teflon screw cap is charged with a solution of B(C6F5)3 (37.9 mg, 1 eq.) and N-isopropylaniline (10.0 mg, 0.074 mmol) in toluene (1 mL). The reaction tube is degassed three times through a freeze-pump-thaw cycle on the vacuum/H2 line and filled with H2 (4 atm) at -196 °C. The reaction bomb is placed in a 110 °C oil bath for 36 h. The toluene is removed under reduced vacuum to yield a white precipitate. The product is washed with pentane (2 x 2 mL) and dried under reduced pressure to give [iPrNH2Cy][HB(C6F5)3] (93 %).
References
- 1)T. Mahdi, Z. M. Heiden, S. Grimme, D. W. Stephan, J. Am. Chem. Soc. 2012, 134, 4088.

- 2)D. W. Stephan, G. Erker, Angew. Chem., Int. Ed. 2010, 49, 46.

应用
Metal-free Hydrogenation of Imines Catalyzed by B(C6F5)3

Typical Procedure: In a glovebox, B(C6F5)3 (18.2 mg, 0.0355 mmol, 20 mol%) is dissolved in dry diisopropylamine (2.5 mL, 1.8 g, 17 mmol) and the solution is added to N-benzylidene-tert-butylamine (28.7 mg, 0.177 mmol, 1 eq.). The resulting solution is transferred to a 25 mL bomb with a sealable Teflon tape and magnetic stirbar. The reaction vessel is sealed, removed from the glovebox and stirred at 100°C for 24 h after which it is cooled to room temperature. The reaction mixture is quenched by the addition of silica followed by elution through a short silica column. The filtrate is concentrated in vacuo to give the desired product.
References
PubMed Literature
技术文章
[产品拾贝] 由三(五氟苯基)硼烷催化的芳香族和芳杂环化合物的氘化反应[产品拾贝] 一种可用于无金属氢化反应的双膦化合物
[研究论文] 低聚硅氧烷的高通量序列可控合成
[研究论文] 三(环己-2,5-二烯-1-基)硅烷: 一种稳定并且易于操作的甲硅烷(SiH4)的替代品
[研究论文] 受阻Lewis酸碱对(FLPs)催化的非金属氢化反应
[研究论文] N-苯基芳环的非金属加氢反应
[研究论文] 通过B(C6F5)3对亚胺进行无金属催化加氢
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱

请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。






