Maximum quantity allowed is 999
请选择数量
CAS RN: 127783-36-2 | 产品编码: P1239
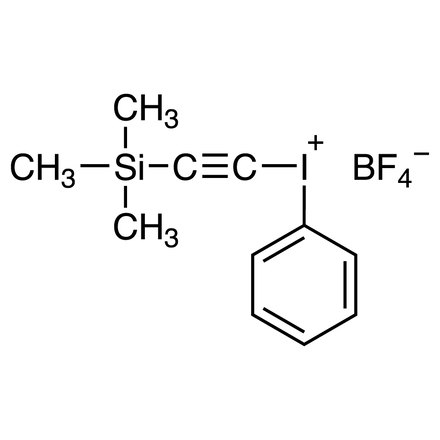
Trimethylsilylethynyl(phenyl)iodonium Tetrafluoroborate
| 产品编码 | P1239 |
纯度/分析方法 
|
>98.0%(T) |
| 分子式/分子量 | C__1__1H__1__4BF__4ISi = 388.03 |
| 外观与形状(20°C) | 固体 |
储存温度 
|
冷冻 (<0°C) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 空气,湿气 (分解),加热 |
包装和容器 
|
1G-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 127783-36-2 |
| Reaxys-RN | 3580345 |
| PubChem物质ID | 87575335 |
| MDL编号 | MFCD00191578 |
技术规格
| Appearance | White to Light yellow to Light orange powder to crystal |
| Purity(Argentometric Titration) | min. 98.0 % |
物性(参考值)
GHS
| 象形图 |


|
| 信号词 | Danger |
| 危险性说明 | H302 : Harmful if swallowed. H314 : Causes severe skin burns and eye damage. |
| 防范说明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dust. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P405 : Store locked up. |
相关法规
运输信息
| UN编号 | UN1759 |
| 类别 | 8 |
| 包装类别 | II |
| HS编码* | 2931.90-000 |
应用
Hypervalent Iodine Reagent Useful for the Chemical Synthesis of Diatomic Carbon

References
- 1) Synthesis of ethynyl(phenyl)iodonium tetrafluoroborate. A new reagent for ethynylation of 1,3-dicarbonyl compounds
- 2) Room-temperature chemical synthesis of C2
- 3) Synthesis of N-(acyloxy)-N-alkynylamides via generation of “C2” from hypervalent alkynyliodane and a weak base
- 4) Routes involving no free C2 in a DFT-computed mechanistic model for the reported room-temperature chemical synthesis of C2
- 5) A combined DFT-predictive and experimental exploration of the sensitivity towards nucleofuge variation in zwitterionic intermediates relating to mechanistic models for unimolecular chemical generation and trapping of free C2 and alternative bimolecular pathways involving no free C2
PubMed Literature