Maximum quantity allowed is 999
CAS RN: 865-47-4 | 产品编码: P1008
Potassium tert-Butoxide
| Appearance | White to Light yellow powder to crystal |
| Purity(Neutralization titration) | min. 97.0 % |
| 熔点 | 240 °C(dec.) |
| 溶解性(可溶于) | 乙醚 |
| 象形图 |


|
| 信号词 | Danger |
| 危险性说明 | H314 : Causes severe skin burns and eye damage. H251 : Self-heating; may catch fire. |
| 防范说明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dusts or mists. P235 + P410 : Keep cool. Protect from sunlight. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P407 : Maintain air gap between stacks/ pallets. P420 : Store away from other materials. P405 : Store locked up. |
| UN编号 | UN3206 |
| 类别 | 4.2 / 8 |
| 包装类别 | II |
| HS编码* | 2905.19-000 |

-
Used Chemicals
-
Procedure
-
To a solution of benzyl alcohol (540 mg, 5.0 mmol), benzonitrile (1.03 g, 10 mmol, 2.0 eq.) in 1,4-dioxane (10 mL) was added potassium tert-butoxide (1.12 g, 10.0 mmol, 2.0 eq.) at r.t. and the mixture was stirred at 130 °C for overnight. The solvent was removed under reduced pressure and the residue was purified by column chromatography (on silica gel, ethyl acetate:hexane = 0:1 - 1:2) several times, giving N-benzylbenzamide as a white solid (513 mg, 49% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by UPLC.
The reaction was carried out using a pressure-resistant test tube.
Purification by column chromatography was performed several times.
-
Analytical Data
-
N-benzylbenzamide
1H NMR (270 MHz, DMSO-d6); δ 9.05 (brt, 1H, J = 5.4 Hz), 7.90 (dd, 1H, J = 7.3, 1.6 Hz), 7.56-7.44 (m, 3H), 7.36-7.32 (m, 4H), 7.25 (q, 1H, J = 7.3 Hz).
-
Lead Reference
-
- Direct Synthesis of Amides from Benzonitriles and Benzylic Alcohols via a KOt‑Bu-Mediated MPV-type Hydrogen Transfer Process
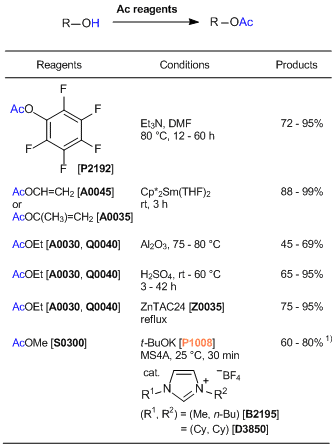
References
- 1)Transesterification/Acylation of Secondary Alcohols Mediated by N-Heterocyclic Carbene Catalysts
- 2)Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.

References
- Potassium tert-Butoxide-Catalyzed Dehydrogenative Si-O Coupling: Reactivity Pattern and Mechanism of an Underappreciated Alcohol Protection
- Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
化学品安全说明书(SDS)
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
示例 CoA
目前没有该产品的 CoA 示例。
分析图谱
很抱歉,您搜索的分析图谱无法提供。








