Maximum quantity allowed is 999
请选择数量
CAS RN: | 产品编码: C2637
| 产品编码 | C2637 |
| 外观与形状(20°C) | 固体 |
| 储存温度 | 室温 (15°C以下阴凉干燥处) |
技术规格
| Appearance | Green to Dark green to Dark blue powder to crystal |
| Loading | 1.2 to 1.5 mmol/g |
| Functionality test | to pass test |
物性(参考值)
GHS
相关法规
运输信息
| HS编码* | 3822.19-000 |
应用
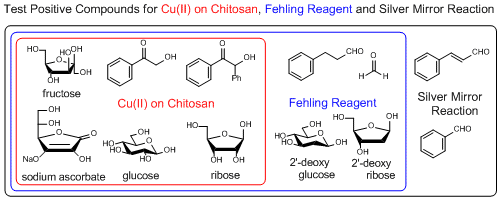
Copper (II) on Chitosan, a reagent for selective discrimination between 2-hydroxy and 2-deoxy sugars, an alternative test reagent for Fehling and Benedict reagents
Copper (II) on chitosan is a chitosan-supported copper (II) (about 1.3 mmol/g) developed by Inoue et al. as a reagent for selective detection of reducible organic compounds. The Cu (II) on chitosan can selectively detect reducible organic compounds bearing structures changeable to ene-diolate (e.g. glucose, ribose) in basic aqueous solution with the formation of copper (I) oxide (Cu2O) as a signal of the reduction by the ene-diolate.

(The color of Cu (II) on chitosan is subject to change to green after opening. But the green-colored Cu (II) on chitosan changes to dark blue which is suitable for this detection in basic aqueous solution, in the same way as a new reagent.)
Typical procedure (detection of a sugar): A test tube (15 mm inside diameters) is charged with copper (II) on chitosan (20 mg) and an aqueous solution of 0.5 mol/L NaOH (1 mL). At this point, the color of copper (II) on chitosan changes from blue to dark blue. Then, an aqueous solution of 10 mmol/L sugar (4 mL) is added, and the mixture is stirred at 70 to 80 °C for 5 min.
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).

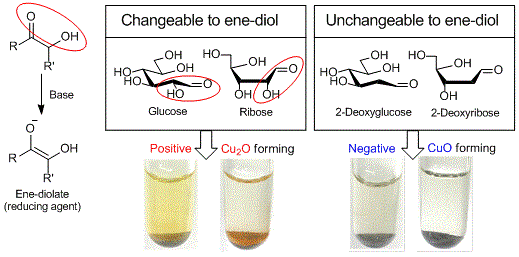
Using Cu (II) on chitosan, needs a lesser amount of Cu (II) than that of Fehling and Benedict reagents and leads to significantly reducing the reagent costs and waste chemicals. In addition, Cu (II) on chitosan is a powdery substance, which is easily handled and free from leaking in carrying and storage. Notably, Cu (II) on chitosan is suitable for science educational experiments such as a test for the detection of a reducing sugar yielded from starch hydrolysis.

References
- S. Ogura, M. Inoue, Kagaku to Kyoiku (Chemical Education) 2013, 61, 86. (Japanese)
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。








