Carbon monoxide (CO) is used as a significant carbonyl group-introducing reagent in organic synthesis. A number of synthetic reactions using CO have been developed. Because CO is a colorless, odorless and toxic gas at room temperature, it is necessary to pay minute attention when using it. For that reason, in the field of the synthetic chemistry using CO, the development of CO surrogate reagents as well as the study of novel synthetic reactions has proceeded.

Manabe et al. have investigated novel CO surrogate agents focused on formic acid derivatives and found that 2,4,6-trichlorophenyl formate (T3121) can act functionally as a CO equivalent.1) This compound is a stable crystalline solid at room temperature and it rapidly decomposes into CO and 2,4,6-trichlorophenol by treatment with a trialkylamine. In situ generated CO is successfully applied to the palladium-catalyzed carbonylation of aryl/alkenyl halides and triflates to give their 2,4,6-trichlorophenol esters. Continuously, the given esters can be transformed into the corresponding carboxylic acid derivatives by the reaction with various nucleophiles. This carbonylation reaction is a highly practical synthetic method since it does not decrease the reaction yields even in gram-scale reactions.2)
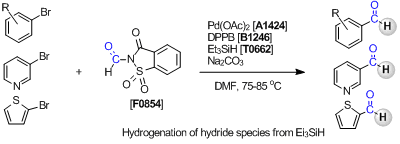
Subsequently, Manabe et al. have developed the reductive carbonylation using N-formylsaccharin (F0854) as a CO equivalent.3) In this reaction, palladium acetate is used as a catalyst and the reaductive carbonylation of aryl bromides is accomplished in combination with triethylsilane as a nucleophile. The hydrogen atom on a formyl group of formed aldehydes is introduced by the addition of the hydride species from triethylsilane.
In a case using 2,4,6-trichlorophenyl formate together with other nucleophiles, the carbonylation reaction tends to be complicated because of the nucleophilicity of 2,4,6-trichlorophenol formed as a by-product. However, in the carbonylation reaction using N-formylsaccharin, hydrosilanes can be directly used as a nucleophile because the nucleophilicity of formed saccharin is sufficiently inert.
References
- 1)T. Ueda, H. Konishi, K. Manabe, Org. Lett. 2012, 14, 5370.

- 2)H. Konishi, T. Ueda, K. Manabe, Org. Synth. 2014, 91, 39.

- 3)T. Ueda, H. Konishi, K. Manabe, Angew. Chem. Int. Ed. 2013, 52, 8611.







![1,1'-Carbonyldiimidazole [Coupling Agent for Peptides Synthesis] 1,1'-Carbonyldiimidazole [Coupling Agent for Peptides Synthesis]](/medias/C0119.jpg?context=bWFzdGVyfHJvb3R8MzE3NTF8aW1hZ2UvanBlZ3xhREl4TDJneFpTODRPVEk1TWpjd09Ea3dOVEkyTDBNd01URTVMbXB3Wnd8ZWMwY2IyNGNkMGNlMzQ0ODA3ZDM1NmRkYjA1ZDBjMDYyOTQzMWI5OTVmMjZiMDI3MGZlMGNhNmEyNTIwNjQyMg)
