Maximum quantity allowed is 999
Please select the quantity
Reagents for the α-Acyloxylation of Aldehydes and Ketones
No.146(August 2011)
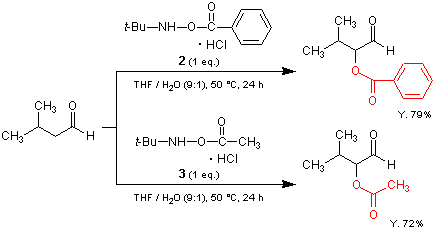
α-Acyloxylated carbonyl compounds are important in organic synthesis, since they are present in many natural products, pharmaceuticals and synthetic intermediates. The three hydroxylamine hydrochlorides described above provide a practical method for the α-acyloxylation of carbonyl compounds. 1 is an effective reagent for the α-benzoyloxylation of both aldehydes and ketones.1,2) An analog with a bulky N-tert-butyl group 2 allows the chemoselective α-benzoyloxylation of aldehydes, since it is completely inert to ketones.1,2) 3 is a reagent for the introduction of the acetyloxy group in aldehydes.3)

Typical Procedure: 1,2)
1,4-Cyclohexanedione mono-ethylene ketal (4.68 g) and 1 (5.63 g) are dissolved in DMSO (7.5 mL) and the resultant mixture is stirred at room temperature for 1 h. The solution is subsequently diluted with ethyl acetate (300 mL) and washed repeatedly with saturated aqueous brine to remove the reaction solvent (5 x 250 mL). The organic fraction is dried over magnesium sulfate and concentrated in vacuo to give a brown solid as a crude product. The crude solid is recrystallized from isopropyl alcohol (5 mL). The resultant solid is washed with ice-cold isopropyl alcohol (50 mL) and ice-cold petroleum ether (50 mL) before being dried under high vacuum (0.07 kPa) for 8 h to yield the 2-benzoyloxylated compound as pale yellow needles (6.38 g, Y. 77%).
1,4-Cyclohexanedione mono-ethylene ketal (4.68 g) and 1 (5.63 g) are dissolved in DMSO (7.5 mL) and the resultant mixture is stirred at room temperature for 1 h. The solution is subsequently diluted with ethyl acetate (300 mL) and washed repeatedly with saturated aqueous brine to remove the reaction solvent (5 x 250 mL). The organic fraction is dried over magnesium sulfate and concentrated in vacuo to give a brown solid as a crude product. The crude solid is recrystallized from isopropyl alcohol (5 mL). The resultant solid is washed with ice-cold isopropyl alcohol (50 mL) and ice-cold petroleum ether (50 mL) before being dried under high vacuum (0.07 kPa) for 8 h to yield the 2-benzoyloxylated compound as pale yellow needles (6.38 g, Y. 77%).
References
- 1)A practical procedure for carbonyl α-oxidation
- T. C. Jones, N. C. O. Tomkinson, Org. Synth. 2007, 84, 233.
- 2)A general method for the α-acyloxylation of carbonyl compounds
- 3)A simple method for the α-oxygenation of aldehydes
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

