Maximum quantity allowed is 999
Please select the quantity
CAS RN: 52462-29-0 | Product Number: D2751
Dichloro(p-cymene)ruthenium(II) Dimer

Purity: >95.0%(T)
Synonyms:
- p-Cymeneruthenium(II) Dichloride Dimer
- Di-μ-chloro-bis[chloro(p-cymene)ruthenium(II)]
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Quantity |
|---|---|---|---|---|
| 1G |
S$88.50
|
4 | ≥40 |
|
| 5G |
S$286.50
|
≥40 | ≥60 |
|
* Please contact our distributors or
TCI
to order TCI products.
* The storage conditions are subject to change without notice.
| Product Number | D2751 |
| Purity / Analysis Method | >95.0%(T) |
| Molecular Formula / Molecular Weight | C__2__0H__2__8Cl__4Ru__2 = 612.38 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 52462-29-0 |
| PubChem Substance ID | 87569153 |
| MDL Number | MFCD00064793 |
Specifications
| Appearance | Light yellow to Brown powder to crystal |
| Purity(Argentometric Titration) | min. 95.0 % |
Properties (reference)
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. H412 : Harmful to aquatic life with long lasting effects. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P273 : Avoid release to the environment. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
Related Laws:
Transport Information:
| H.S.code* | 2843.90-000 |
Application
Ruthenium-catalyzed Direct C―H Amidation of Arenes

Typical Procedure:
Acetophenone (0.4 mmol), p-toluenesulfonyl azide (0.2 mmol), [RuCl2(p-cymene)]2 (4.9 mg, 4 mol%), AgNTf2 (12.5 mg, 16 mol%), NaOAc (3.3 mg, 20 mol%), and 1,2-dichloroethane (0.5 mL) are added to a screw-capped vial equipped with a spinvane triangular-shaped Teflon stirbar. The reaction mixture is stirred in a preheated oil bath at 80 °C for 12 h and then cooled to room temperature, filtered through a pad of Celite, and washed with ethyl acetate (10 mL×3). The filtrate is concentrated under reduced pressure and the residue is purified by chromatography on silica gel (n-hexane : EtOAc = 3 : 1) to give the desired product (56.1 mg, Y. 97%).
Acetophenone (0.4 mmol), p-toluenesulfonyl azide (0.2 mmol), [RuCl2(p-cymene)]2 (4.9 mg, 4 mol%), AgNTf2 (12.5 mg, 16 mol%), NaOAc (3.3 mg, 20 mol%), and 1,2-dichloroethane (0.5 mL) are added to a screw-capped vial equipped with a spinvane triangular-shaped Teflon stirbar. The reaction mixture is stirred in a preheated oil bath at 80 °C for 12 h and then cooled to room temperature, filtered through a pad of Celite, and washed with ethyl acetate (10 mL×3). The filtrate is concentrated under reduced pressure and the residue is purified by chromatography on silica gel (n-hexane : EtOAc = 3 : 1) to give the desired product (56.1 mg, Y. 97%).
References
Application
Ruthenium-Catalyzed Isoquinolone Synthesis
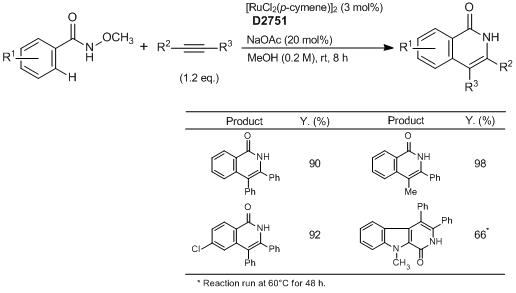
Typical Procedure:
A mixture of N-methoxybenzamide (0.50 mmol, 1.0 eq.), the alkyne (if solid) (0.60 mmol, 1.2 eq.), [RuCl2(p-cymene)]2 (9.2 mg, 0.015 mmol, 3.0 mol%) and NaOAc (8.2 mg, 0.10 mmol, 20.0 mol%) are placed in a Schlenk tube equipped with a stir bar. Dry MeOH (2.5 mL) is added (followed by the alkyne if it is a liquid) and the mixture is stirred at room temperature for 8 h under an argon atmosphere. Afterwards, the mixture is diluted with CH2Cl2 and transferred to a round-bottomed flask. Silica is added to the flask and volatiles are evaporated under reduced pressure. The purification is performed by flash column chromatography on silica gel (EtOAc : petroleum ether=1:5 to 1:1, typically) to give the corresponding pure products.
A mixture of N-methoxybenzamide (0.50 mmol, 1.0 eq.), the alkyne (if solid) (0.60 mmol, 1.2 eq.), [RuCl2(p-cymene)]2 (9.2 mg, 0.015 mmol, 3.0 mol%) and NaOAc (8.2 mg, 0.10 mmol, 20.0 mol%) are placed in a Schlenk tube equipped with a stir bar. Dry MeOH (2.5 mL) is added (followed by the alkyne if it is a liquid) and the mixture is stirred at room temperature for 8 h under an argon atmosphere. Afterwards, the mixture is diluted with CH2Cl2 and transferred to a round-bottomed flask. Silica is added to the flask and volatiles are evaporated under reduced pressure. The purification is performed by flash column chromatography on silica gel (EtOAc : petroleum ether=1:5 to 1:1, typically) to give the corresponding pure products.
References
PubMed Literature
Articles/Brochures
TCIMAIL
[TCIMAIL No.107] Arene Ruthenium(II) Complexes[Research Articles] Ruthenium-catalyzed Direct C-H Amidation of Arenes
[Research Articles] Ruthenium-Catalyzed Isoquinolone Synthesis
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





