Product Document Searching Made Easy by 2D Code! | TCI Chemistry News October 2025 | [TCIPracticalExample] Di-Cbz Guanidinylation of Amine... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 929896-28-6 | Product Number: B4195
Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium
![Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium Chemical Structure of Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium](/medias/B4195.jpg?context=bWFzdGVyfHJvb3R8NjUyNzd8aW1hZ2UvanBlZ3xhRGsxTDJnMFlpODRPVEl3TWpVNE1UZ3pNVGs0TDBJME1UazFMbXB3Wnd8NTM3ZDVkNGU3NWI1NjY5Y2VhNjM2YzAzODdmM2U3MjhhOGVhNjM1ZGM0MTExNGM2MWJmNjgyYjE5NWFjM2YyNQ)
Purity:
Synonyms:
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time | Quantity |
Shipping Information

|
|---|---|---|---|---|---|---|
| 10MG |
S$363.00
|
0 | 0 | Contact Us |
|
* Please contact our distributors or
TCI
to order TCI products.
* The storage conditions are subject to change without notice.
| Product Number | B4195 |
| Molecular Formula / Molecular Weight | C__2__4H__3__5N__2O__7Rh = 566.46 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 929896-28-6 |
| PubChem Substance ID | 253659893 |
Specifications
| Appearance | Light yellow to Yellow to Orange powder to crystal |
| Elemental analysis(Nitrogen) | 4.40 to 5.20 % |
| NMR | confirm to structure |
Properties (reference)
GHS
Related Laws:
Transport Information:
| H.S.code* | 2843.90-000 |
Application
Pincer Type Rh Complex Catalyzed Asymmetric Diboration of Terminal Alkenes and Their Conversion to the Optically Active 1,2-Diols
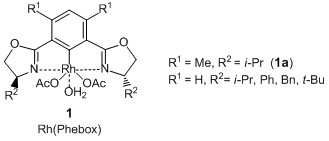

Nishiyama et al. have reported that optically active 1,2-diols are given by the pincer type Rh complexes [Rh(Phebox), 1] catalyzed asymmetric 1,2-diboration of terminal alkenes and subsequent oxidation. According to their results, the asymmetric diboration of 4-chlorostyrene with a diboron ester proceeds with high enantioselectivity by using a rhodium complex coordinated with a pincer type ligand (1a), and the desired 1,2-diborylated product is formed. Subsequent oxidation of it with sodium peroxoborate in THF-water gives the corresponding 1,2-diol. In this reaction, allyl ethers and allyl anilines can be transformed into the related optically active 1,2-diols. In addition, when using trans 1,3-dienes as reactants, terminal alkene moieties are selectively reacted and the optically active allylic alcohols are given. Thus, this asymmetric diboration using 1 is an efficient synthetic method to produce the optically active 1,2-diols.
References
- Asymmetric diboration of terminal alkenes with a rhodium catalyst and subsequent oxidation: enantioselective synthesis of optically active 1,2-diols
PubMed Literature
Product Articles
[TCIMAIL No.160] Pincer Type Rh Complex Catalyzed Asymmetric Diboration of Terminal Alkenes and Their Conversion to the Optically Active 1,2-DiolsProduct Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.


![Thumbnail of Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium](/medias/B4195.jpg?context=bWFzdGVyfHJvb3R8MTc3NDB8aW1hZ2UvanBlZ3xhR0UxTDJobU1pODRPVE16TmpjeU1Ea3hOamM0TDBJME1UazFMbXB3Wnd8YjUzYTM1YjZlZGNhYmU3OGMxM2EyOTkyNWQyYWZjNzc3NDBhMDEwZGZkZmFmOTNhOTVjOWY0NjQzY2YzMmY5MA)
![Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium](/_ui/responsive/theme-tci/images/missing_product_zoom.webp)

