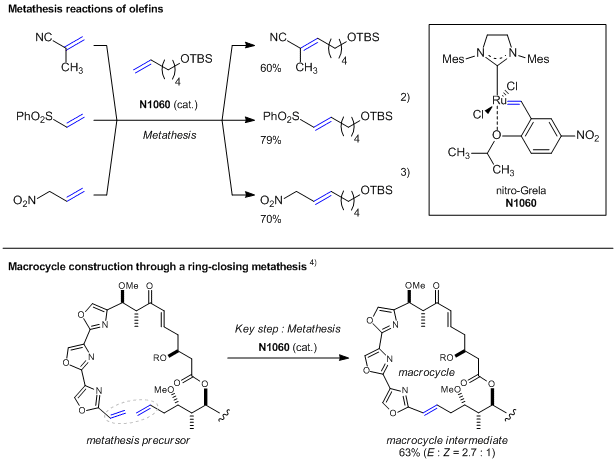
Nitro-Grela is one of Hoveyda-type metathesis catalysts and is effectively used for
trans-selective olefin metathesis reactions. This complex has an isopropoxy group on a phenylvinylidene moiety offering coordination to a ruthenium metal while bearing an electron-withdrawing nitro group. From these two structural relations, the complex shows the compatible properties of stabilization of the Ru-O coordination bond and the nitro group-induced increase of a dissociation ability, which resultantly shows a larger substrate scope while maintaining the activity of typical ruthenium vinylidene complexes.
1) By using the catalyst, metathesis reactions with a cyano or sulfonyl group-substituted alkenes,
2) and nitro alkenes bearing an active methylene moiety
3) can be successfully performed. In addition, it shows high applicabilities to macrocycle constructions in natural compound syntheses.
4)