Maximum quantity allowed is 999
Please select the quantity
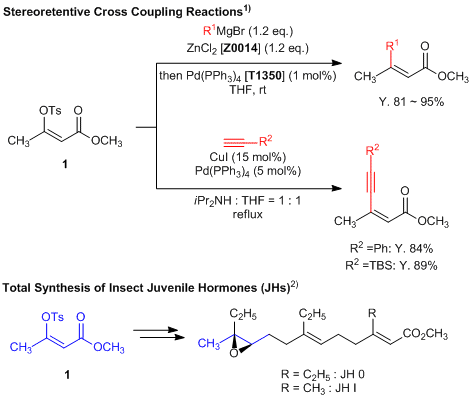
Enol Tosylate for Stereoretentive Cross Coupling Reactions
Methyl (Z)-3-(p-toluenesulfonyloxy)but-2-enoate (1) is a useful building block featuring an enol tosylate moiety which can be utilized in stereoselective cross coupling reactions. In this instance, treatment of 1 under either Negishi or Sonogoshira conditions affords two coupled products substituted at the tosyl position.1) This reaction has the advantage of stereoretentively proceeding of alkene moiety. Furthermore, total synthesis of insect juvenile hormones from 1 has been reported.2)
Related Products
References
- 1)General, Robust, and Stereocomplementary Preparation of β-Ketoester Enol Tosylates as Cross-Coupling Partners Utilizing TsCl-N-Methylimidazole Agents
- 2)Stereoselective Total Syntheses of Insect Juvenile Hormones JH 0 and JH I