Maximum quantity allowed is 999
Please select the quantity
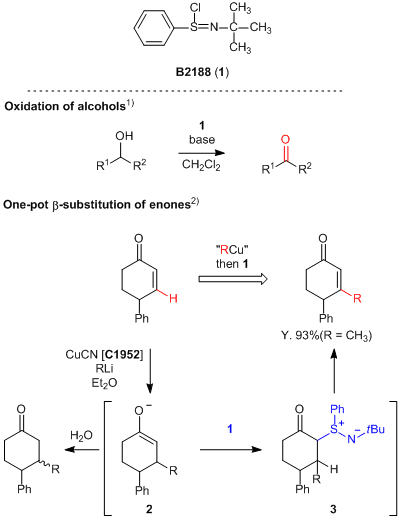
Effective Oxidant for the Alcohol Oxidation and β-Alkyl Enone Synthesis
N-tert-Butylbenzenesulfinimidoyl chloride (1), developed by Matsuo and
Mukaiyama et al., is an oxidant and is efficiently used for the oxidation of alcohols at room temperature
with ease of handling.
1)
1 effectively oxidizes not only typical alcohols but also benzylic and allylic alcohols to aldehydes
without complications. Furthermore, 1 is used for the reaction to introduce alkyl groups into the
β-position of enones in one pot.
2)
In this reaction, the 1,4-addition of alkyl copper reagents to enones successfully proceeds to form the
enolate 2, and the subsequent reaction with 1 affords the desired β-alkyl enones via the
formation of an intermediate 3. In this way, 1 is easy-to-handle and effective in alcohol oxidations
and β-alkyl substitutions of enones, so that it is expected to be widely applied for various organic
syntheses such as natural products.
Related Products
- B2188
- N-tert-Butylbenzenesulfinimidoyl Chloride (*This product is discontinued.)
References
- 1) A New and Efficient Method for Oxidation of Various Alcohols by Using N-tert-Butyl Phenylsulfinimidoyl Chloride
- 2) One-pot β-substitution of enones with alkyl groups to β-alkyl enones